All Photos(1)
About This Item
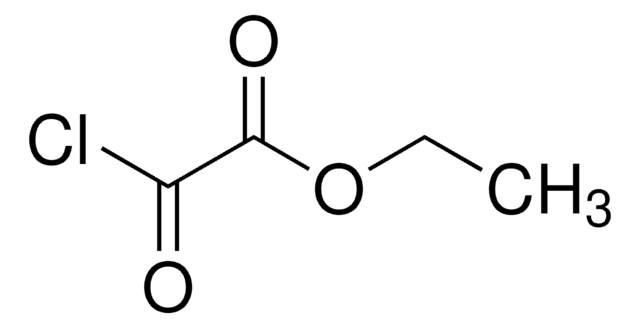
Linear Formula:
CH3CHClCOCH3
CAS Number:
Molecular Weight:
106.55
Beilstein:
385637
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.421 (lit.)
bp
114-117 °C (lit.)
density
1.055 g/mL at 25 °C (lit.)
SMILES string
CC(Cl)C(C)=O
InChI
1S/C4H7ClO/c1-3(5)4(2)6/h3H,1-2H3
InChI key
OIMRLHCSLQUXLL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
3-Chloro-2-butanone reacts with 1,4-dianion of acetophenone N-ethoxycarbonylhydrazone to yield pyrazoline derivatives.
Application
3-Chloro-2-butanone was used in production of chiral alcohols from acetophenone derivatives, β-ketoesters and N-Boc-3-pyrrolidinone by recombinant E. coli cells. It was used in the synthesis of carbene precursor, 3-aryl-4,5-dimethylthiazolium chloride.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
82.4 °F - closed cup
Flash Point(C)
28 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Georgios C Vougioukalakis et al.
Journal of the American Chemical Society, 130(7), 2234-2245 (2008-01-29)
A new family of ruthenium-based olefin metathesis catalysts bearing a series of thiazole-2-ylidene ligands has been prepared. These complexes are readily accessible in one step from commercially available (PCy3)2Cl2Ru=CHPh or (PCy3)Cl2Ru=CH(o-iPrO-Ph) and have been fully characterized. The X-ray crystal structures
Reaction of α-chloroketones with 1, 4-dianion of acetophenone n-ethoxy-carbonylhydrazone.
Matsumura N, et al.
Tetrahedron Letters, 25(40), 4529-4532 (1984)
Nobuya Itoh et al.
European journal of biochemistry, 269(9), 2394-2402 (2002-05-03)
Phenylacetaldehyde reductase (PAR) produced by styrene-assimilating Corynebacterium strain ST-10 was used to synthesize chiral alcohols. This enzyme with a broad substrate range reduced various prochiral aromatic ketones and beta-ketoesters to yield optically active secondary alcohols with an enantiomeric purity of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service