All Photos(1)
About This Item
Empirical Formula (Hill Notation):
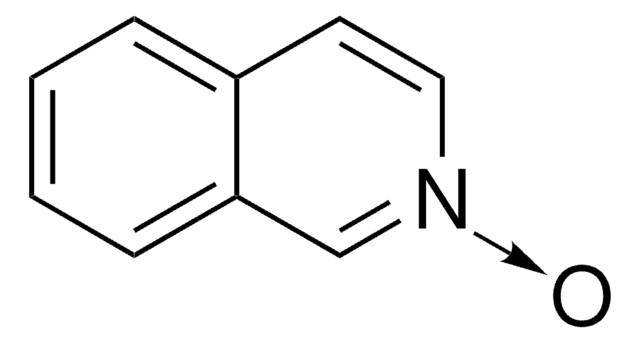
C9H7NO · xH2O
CAS Number:
Molecular Weight:
145.16 (anhydrous basis)
Beilstein:
5989589
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
52-55 °C (lit.)
SMILES string
[H]O[H].[O-][n+]1cccc2ccccc12
InChI
1S/C9H7NO.H2O/c11-10-7-3-5-8-4-1-2-6-9(8)10;/h1-7H;1H2
InChI key
CUSWDTBBCIXCRB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Quinoline N-oxide hydrate forms complexes with lanthanide chloride.
Application
Quinoline N-oxide hydrate was used in quantitative determination of nitrones using trifluoroacetic anhydride-sodium iodide reagent.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The application of trifluoroacetic anhydride-sodium iodide (TFAA-I) system for quantitative determination of nitrones.
Clesielski W, et al.
Canadian Journal of Chemistry, 68(5), 679-684 (1990)
Lanthanide chloride complexes with quinoline-n-oxide.
Kingston JV, et al.
J. Inorg. Nucl. Chem., 31(10), 3181-3185 (1969)
Oleg V Larionov et al.
Organic letters, 16(3), 864-867 (2014-01-15)
A one-step transformation of heterocyclic N-oxides to 2-alkyl-, aryl-, and alkenyl-substituted N-heterocycles is described. The success of this broad-scope methodology hinges on the combination of copper catalysis and activation by lithium fluoride or magnesium chloride. The utility of this method
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service