SBR00015
AEBSF Ready Made Solution
Synonym(s):
4-(2-Aminoethyl)benzenesulfonyl fluoride hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
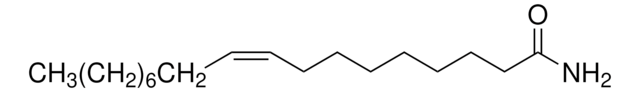
Empirical Formula (Hill Notation):
C8H10FNO2S · HCl
CAS Number:
Molecular Weight:
239.69
MDL number:
UNSPSC Code:
12352202
NACRES:
NA.85
Recommended Products
Assay
≥97%
Quality Level
form
liquid
storage condition
protect from light
shipped in
dry ice
storage temp.
−20°C
InChI
1S/C8H10FNO2S.ClH/c9-13(11,12)8-3-1-7(2-4-8)5-6-10;/h1-4H,5-6,10H2;1H
InChI key
WRDABNWSWOHGMS-UHFFFAOYSA-N
Application
This solution is provided at 100 mM in water. The stock solution may be diluted 1:100 for a 1 mM working concentration. Recommended concentrations for use are 0.1-1.0 mM.
Biochem/physiol Actions
AEBSF is a specific irreversible serine protease inhibitor. It inhibits proteases like chymotrypsin, kallikrein, plasmin, thrombin, trypsin, and related thrombolytic enzymes. AEBSF is an excellent alternative to available serine protease inhibitors as it offers superior inhibitory activity and much lower toxicological risk.
Other Notes
The stability of AEBSF decreases rapidly at higher temperatures and at a pH greater than 7.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Toru Hosoi et al.
European journal of pharmacology, 554(1), 8-11 (2006-11-18)
Endoplasmic reticulum stress contributes to several diseases such as neurodegenerative disorders and diabetes. In the previous report, we found that phosphatidylinositol 3-kinase (PI3K) down-regulation is important for inducing CHOP expression, an endoplasmic reticulum stress-induced transcription factor. In the present study
G Lunn et al.
Applied biochemistry and biotechnology, 48(2), 57-59 (1994-08-01)
Five enzyme inhibitors (phenylmethylsulfonyl fluoride, 4-amidinophenylmethanesulfonyl fluoride, 4-(2-aminoethyl)benzenesulfonyl fluoride, N alpha-p-tosyl-L-lysine chloromethyl ketone, and N-tosyl-L-phenylalanine chloromethyl ketone) in buffer, DMSO, or stock solutions were completely degraded by adding 1M NaOH and the final reaction mixtures were not mutagenic. The stability
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service