D9891
Doxycycline hyclate
Synonym(s):
6-Desoxy-5-hydroxytetracycline hydrochloride hemihydrate hemiethanolate, Doxycycline hydrochloride hemiethanolate hemihydrate
About This Item
Recommended Products
biological source
synthetic
Quality Level
Assay
≥93.5% (HPLC)
95.0-102.0% anhydrous basis (ethanol free based)
form
powder
storage condition
(Tightly closed. Dry. )
color
yellow to yellow-green
antibiotic activity spectrum
Gram-negative bacteria
Gram-positive bacteria
mycoplasma
parasites
Mode of action
protein synthesis | interferes
storage temp.
2-8°C
SMILES string
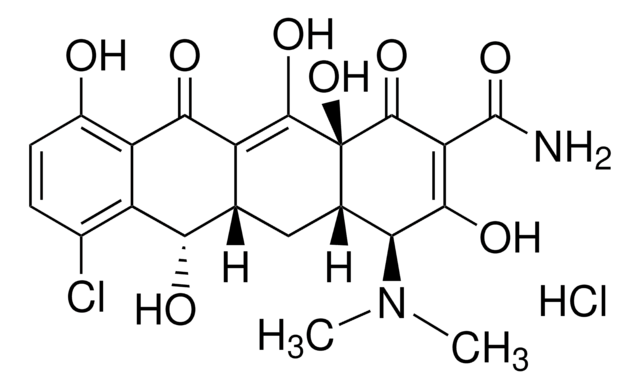
Cl[H].Cl[H].[H]O[H].CCO.C[C@@H]1C2[C@H](O)C3[C@H](N(C)C)C(O)=C(C(N)=O)C(=O)[C@@]3(O)C(O)=C2C(=O)c4c(O)cccc14.C[C@@H]5C6[C@H](O)C7[C@H](N(C)C)C(O)=C(C(N)=O)C(=O)[C@@]7(O)C(O)=C6C(=O)c8c(O)cccc58
InChI
1S/2C22H24N2O8.C2H6O.2ClH.H2O/c2*1-7-8-5-4-6-9(25)11(8)16(26)12-10(7)17(27)14-15(24(2)3)18(28)13(21(23)31)20(30)22(14,32)19(12)29;1-2-3;;;/h2*4-7,10,14-15,17,25,27-29,32H,1-3H3,(H2,23,31);3H,2H2,1H3;2*1H;1H2/t2*7-,10?,14?,15-,17-,22-;;;;/m00..../s1
InChI key
HALQELOKLVRWRI-ZVACAFRPSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Beyond its antimicrobial effects, Doxycycline Hyclate serves as an inhibitor of matrix metalloproteinases (MMPs), enzymes crucial for collagen breakdown in connective tissues. Studies on corneal erosion and rheumatoid arthritis showcase its significant reduction in MMP-9 and MMP-8 levels, respectively. This MMP-inhibiting property helps preserve collagen integrity, preventing excessive tissue degradation and supporting tissue repair.
In cell biology, Doxycycline Hyclate impacts smooth muscle cells, enhancing adhesion and influencing the reorganization of fibrillar collagen matrices. Its influence on apicoplast gene expression in Plasmodium falciparum, the malaria-causing parasite, adds a dimension of significance in infectious disease research. Additionally, the inhibition of tissue formation in rat studies contributes to its relevance in understanding tissue development.
Moreover, Doxycycline Hyclate holds potential in metabolomics research, considering its multi-faceted effects on various cellular processes. In the broader context of biochemical research, its versatile actions make it a valuable tool for investigating molecular mechanisms and signaling pathways.
Application
Biochem/physiol Actions
Activity Spectrum: Effective against a broad spectrum inhibitor of matrix metalloproteinases in vivo.
Features and Benefits
- High-quality antibiotic suitable for multiple research applications
- Ideal for Cell Biology, Metabolomics, and Biochemical research.
Other Notes
also commonly purchased with this product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service