All Photos(1)
About This Item
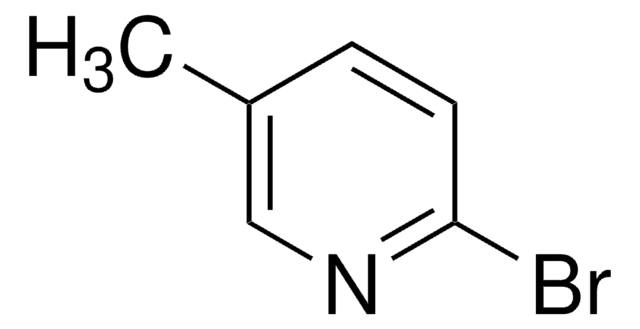
Empirical Formula (Hill Notation):
C11H11NO
Molecular Weight:
173.21
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
reaction suitability
reagent type: oxidant
mp
104-109 °C
storage temp.
2-8°C
SMILES string
CCC1=CC=CC2=C1[N+]([O-])=CC=C2
InChI
1S/C11H11NO/c1-2-9-5-3-6-10-7-4-8-12(13)11(9)10/h3-8H,2H2,1H3
InChI key
LZEBWZDDNNWJJK-UHFFFAOYSA-N
Application
Reactant for the preparation of:
- α, β-unsaturated enones with catalytic amounts of Gold complexes
- Synthesis of azetidines (4-membered nitrogen containing heterocycles) through an intramolecular oxidative cyclization in the presence of catalytic amounts of Gold complexes
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A flexible and stereoselective synthesis of azetidin-3-ones through gold-catalyzed intermolecular oxidation of alkynes.
Longwu Ye et al.
Angewandte Chemie (International ed. in English), 50(14), 3236-3239 (2011-03-08)
Biao Lu et al.
Journal of the American Chemical Society, 132(40), 14070-14072 (2010-09-22)
Gold-catalyzed intermolecular oxidations of internal alkynes have been achieved with high regioselectivities using 8-alkylquinoline N-oxides as oxidants and in the absence of acid additives. Synthetically versatile α,β-unsaturated carbonyls are obtained in good to excellent yields and with excellent E-selectivities. A
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
iridium(III) 97%](/deepweb/assets/sigmaaldrich/product/structures/309/053/0823f035-245c-433d-b033-2eca2d931c67/640/0823f035-245c-433d-b033-2eca2d931c67.png)