All Photos(1)
About This Item
Empirical Formula (Hill Notation):
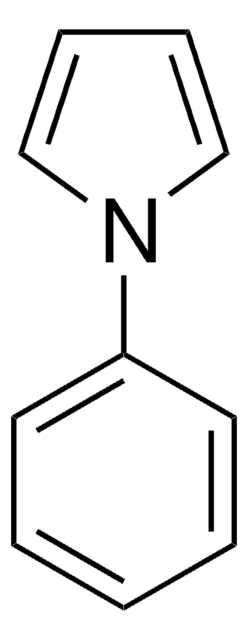
C11H11N
CAS Number:
Molecular Weight:
157.21
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.5685 (lit.)
bp
90 °C/0.9 mmHg (lit.)
bulk density
1.020 g/mL
SMILES string
C(c1ccccc1)n2cccc2
InChI
1S/C11H11N/c1-2-6-11(7-3-1)10-12-8-4-5-9-12/h1-9H,10H2
InChI key
FNEQHKCQXDKYEO-UHFFFAOYSA-N
General description
N-benzylpyrrole can be synthesized from N-benzylpyrrolidine via oxidation with 2-iodoxybenzoic acid(IBX) in presence of β-cyclodextrin in aqueous medium.
Application
N-benzylpyrrole may be used in the synthesis of the following:
- (E,Z)-3-(7,8-dimethoxy-5H-pyrrolo[2,1-a]isoindol-3-yl)-N,N-diethylacrylamide
- (E,Z)-7,8-dimethoxy-3-styryl-5H-pyrrolo[2,1-a]isoindole
- pyrroloisoquinolines
- (Z)-2-(7,8-dimethoxypyrrolo[1,2-b]isoquinolin-10-ylidene)-N,N-diethylacetamide
- (E,Z)-10-benzylidene-7,8-dimethoxy-5,10-dihydropyrrolo- [1,2-b]isoquinoline
- (E,Z)-7,8-dimethoxy-10-(2-methoxyvinyl)-5,10-dihydropyrrolo[1,2-b]isoquinoline
- (Z)-7,8-dimethoxy-10-(methoxymethylene)-5,10-dihydropyrrolo[1,2-b]isoquinoline
- 2-cyano-N-benzylpyrroles
- 3-cyano-N-benzylpyrroles
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
"Metal-Free One-Pot Conversion of Electron-Rich Aromatics into Aromatic Nitriles"
Ushijima S and Togo H
Synlett, 2010(07), 1067-1070 (2010)
"Intramolecular Palladium-Catalyzed Direct Arylation vs. Heck Reactions: Synthesis of Pyrroloisoquinolines and Isoindoles"
Lage S, et al.
Advanced Synthesis & Catalysis, 351(14 - 15), 2460-2468 (2009)
"o-Iodoxybenzoic acid (IBX): a versatile reagent for the synthesis of N-substituted pyrroles mediated by ?-cyclodextrin in water"
Murthy N.S and Nageswar DVY
Tetrahedron Letters, 52(34), 4481-4484 (2001)
Hannah F Dugdale et al.
Molecular and cellular biochemistry, 444(1-2), 109-123 (2017-12-01)
Glucose restriction (GR) impairs muscle cell differentiation and evokes myotube atrophy. Resveratrol treatment in skeletal muscle cells improves inflammatory-induced reductions in skeletal muscle cell differentiation. We therefore hypothesised that resveratrol treatment would improve muscle cell differentiation and myotube hypertrophy in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service