556327
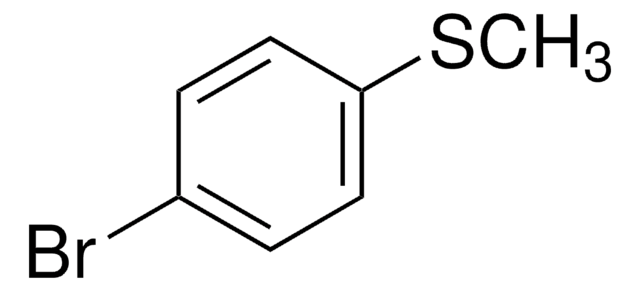
4-Bromophenyl methyl sulfone
97%
Synonym(s):
1-Bromo-4-(methylsulfonyl)benzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrC6H4SO2CH3
CAS Number:
Molecular Weight:
235.10
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
103-107 °C (lit.)
SMILES string
CS(=O)(=O)c1ccc(Br)cc1
InChI
1S/C7H7BrO2S/c1-11(9,10)7-4-2-6(8)3-5-7/h2-5H,1H3
InChI key
FJLFSYRGFJDJMQ-UHFFFAOYSA-N
Related Categories
Application
4-Bromophenyl methyl sulfone may be used to synthesize:
4-Bromophenyl methyl sulfone (1-bromo-4-(methylsulfonyl)benzene) can undergo coupling reaction with benzene sulfonamide in the presence of copper(I)iodide to form the corresponding N-aryl sulfonamide.
- biaryl methyl sulfones
- 5-[[-4-(methylsulfonyl)phenyl]thio]thiophene-2-sulfonamide
- 1-[4-(methylsulfonyl)phenyl]-1H-pyrazole (Hmsppz)
- DuP 697 via reaction with (5-chloro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)thiophen-2-yl)trimethylsilane
4-Bromophenyl methyl sulfone (1-bromo-4-(methylsulfonyl)benzene) can undergo coupling reaction with benzene sulfonamide in the presence of copper(I)iodide to form the corresponding N-aryl sulfonamide.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
?Copper-catalyzed N-arylation of sulfonamides with aryl bromides under mild conditions?
Wang X, et al.
Tetrahedron Letters, 53, 7?10-7?10 (2012)
?Divergent synthesis of 2,3,5-substituted thiophenes by C-H activation/borylation/suzuki coupling?
Kallepalli.AV, et al.
Heterocycles, 80(2), 1429 - 1448 (2010)
Cooperativity and steric hindrance: important factors in the binding of a-cyclodextrin with para-substituted aryl alkyl sulfides, sulfoxides and sulfones.
Davies DM and Deary ME.
J. Chem. Soc. Perkin Trans. II, 7, 1287-1294 (1995)
Daniel Tordera et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 19(26), 8597-8609 (2013-05-08)
A new approach to obtain green-emitting iridium(III) complexes is described. The synthetic approach consists of introducing a methylsulfone electron-withdrawing substituent into a 4-phenylpyrazole cyclometalating ligand in order to stabilize the highest-occupied molecular orbital (HOMO). Six new complexes have been synthesized
I T Barnish et al.
Journal of medicinal chemistry, 24(8), 959-964 (1981-08-01)
A series of 5-(arylthio)-, 5-(arylsulfinyl)-, and 5-(arylsulfonyl)thiophene-2-sulfonamides is described and anticonvulsant activities are listed for the compounds. In most cases, the sulfones had the highest activity and the sulfides the least. Sulfones with 3- or 4-halo substituents generally had the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service