All Photos(1)
About This Item
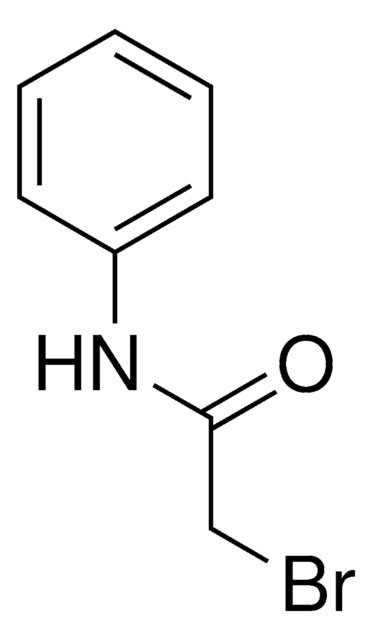
Linear Formula:
CH3CONHC6H4Br
CAS Number:
Molecular Weight:
214.06
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
mp
96.5-100.5 °C (lit.)
SMILES string
CC(=O)Nc1ccccc1Br
InChI
1S/C8H8BrNO/c1-6(11)10-8-5-3-2-4-7(8)9/h2-5H,1H3,(H,10,11)
InChI key
VOBKUOHHOWQHFZ-UHFFFAOYSA-N
General description
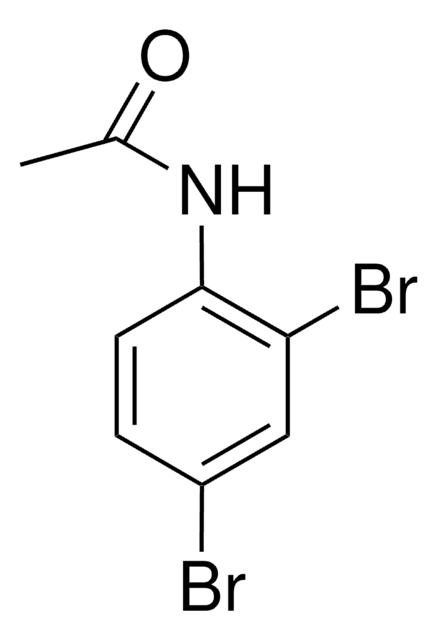
2′-Bromoacetanilide can be prepared from aniline via bromoacetylation. 2-Bromoacetanilide undergoes bromination in the presence of acetic acid to afford 2,4′-dibromoacetanilide.
Application
2′-Bromoacetanilide (2-bromoacetanilide) may be used to synthesize:
- 2-iodophenylmethylphosphinic acid
- substituted biaryl acetamide compounds
- benzisoxazolo[2,3-a]pyridinium tetrafluoroborates
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Palladium-catalyzed direct arylation of pyridine N-oxide with 2-bromoacetanilides. Synthesis of benzisoxazolo [2, 3-a] pyridinium tetrafluoroborates.
Myers JT and Hanna JM.
Tetrahedron Letters, 53(2), 612-615 (2012)
Extension of the Smiles rearrangement. Displacement of an aromatic amide group by an amine nitrogen.
Gilman NW, et al.
The Journal of Organic Chemistry, 38(2), 373-377 (1973)
Synthesis of chelating compounds to be used as potential bone seekers.
G Shtacher et al.
Journal of medicinal chemistry, 9(2), 197-203 (1966-03-01)
Synthesis and molecular structure of 1, 3-dihydro-1-hydroxy-3-methyl-1, 2, 3-benziodoxaphosphole 3-oxide.
Balthazor TM, et al.
The Journal of Organic Chemistry, 43(23), 4538-4540 (2978)
W C Peter Tsang et al.
The Journal of organic chemistry, 73(19), 7603-7610 (2008-09-03)
The development of a new method for the assembly of unsymmetrical carbazoles is reported. The strategy involves the selective intramolecular functionalization of an arene C-H bond and the formation of a new arene C-N bond. The substitution pattern of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service