All Photos(1)
About This Item
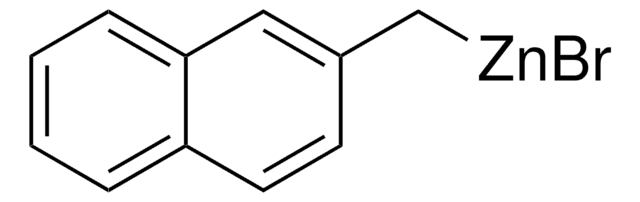
Empirical Formula (Hill Notation):
C4H3BrSZn
CAS Number:
Molecular Weight:
228.43
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
concentration
0.5 M in THF
density
0.973 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
Br[Zn]c1cccs1
InChI
1S/C4H3S.BrH.Zn/c1-2-4-5-3-1;;/h1-3H;1H;/q;;+1/p-1
InChI key
KBBMRTYVFOYNIW-UHFFFAOYSA-M
Related Categories
Application
2-Thienylzinc bromide can be used as:
- A starting material in the synthesis of heteroaryl sulfonamides by reacting with 2,4,6-trichlorophenyl chlorosulfate.
- A substrate in the synthesis of naphthacenodithiophene derived polymers, which are used in transistor and solar cell devices.
- An intermediate in the preparation of isoquinoline derived AICARFT inhibitors.
Legal Information
Product of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-0.0 °F - closed cup
Flash Point(C)
-17.78 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Naphthacenodithiophene based polymers-new members of the acenodithiophene family exhibiting high mobility and power conversion efficiency
Knall A-C, et al.
Advances in Functional Materials, 26(38), 6961-6969 (2016)
Discovery of N-(6-Fluoro-1-oxo-1, 2-dihydroisoquinolin-7-yl)-5-[(3 R)-3-hydroxypyrrolidin-1-yl] thiophene-2-sulfonamide (LSN 3213128), a Potent and Selective Nonclassical Antifolate Aminoimidazole-4-carboxamide Ribonucleotide Formyltransferase (AICARFT) Inhibitor Effective at Tumor Suppression in a Cancer Xenograft Model
Fales KR, et al.
Journal of Medicinal Chemistry, 60(23), 9599-9616 (2017)
Synthesis of Heteroaryl Sulfonamides from Organozinc Reagents and 2, 4, 6-Trichlorophenyl Chlorosulfate
Colombe JR, et al.
Organic Letters, 17(12), 3170-3173 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) >95% in F+ active](/deepweb/assets/sigmaaldrich/product/structures/206/487/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d/640/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d.png)