494968
Thianaphthene-3-carboxaldehyde
95%
Synonym(s):
Benzo[b]thiophene-3-carboxaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C9H6OS
CAS Number:
Molecular Weight:
162.21
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
bp
166 °C/20 mmHg (lit.)
mp
53-57 °C (lit.)
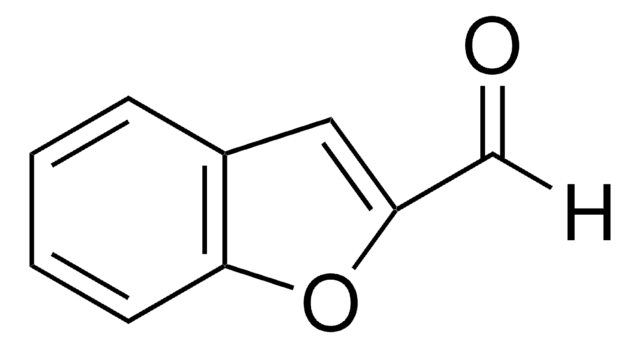
SMILES string
O=Cc1csc2ccccc12
InChI
1S/C9H6OS/c10-5-7-6-11-9-4-2-1-3-8(7)9/h1-6H
InChI key
WDJLPQCBTBZTRH-UHFFFAOYSA-N
General description
Thianaphthene-3-carboxaldehyde, also known as benzo[b]thiophene-3-carboxaldehyde, can be synthesized from 3-methyl-benzo[b]thiophene. It undergoes phosphine-free palladium coupling with aryl halides to form 2-arylbenzo[b]thiophenes.
Application
Thianaphthene-3-carboxaldehyde (Benzo[b]thiophene-3-carboxaldehyde) may be used as a starting material in the multi-step synthesis of anthra[2,3-b:7,6-b′]bis[1benzothiophenes (ABBTs).
It may be used in the synthesis of :
It may be used in the synthesis of :
- 6-(N,N-dimethylamino)-2-(benzo[b]thiophen-3-yl)quinazolin-4-one
- 6-(pyrrolidin-1-yl)-2-(benzo[b]thiophen-3-yl)quinazolin-4-one
- (Z)-2-(benzo[b]thiophen-3-ylmethylene)-1-azabicyclo[2.2.2]octan-3-one
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M J Hour et al.
British journal of pharmacology, 169(7), 1574-1586 (2013-05-04)
Our previous study demonstrated that 6-(pyrrolidin-1-yl)-2-(3-methoxyphenyl)quinazolin-4-one (HMJ38) was a potent anti-tubulin agent. Here, HMJ38 was used as a lead compound to develop more potent anti-cancer agents and to examine the anti-cancer mechanisms. Using computer-aided drug design, 2-aryl-6-substituted quinazolinones (MJ compounds)
An efficient phosphine-free palladium coupling for the synthesis of new 2-arylbenzo[b]thiophenes.
Chabert JFD, et al.
Tetrahedron, 60(14), 3221-3230 (2004)
Benzo[b]thiophene derivatives. VIII. Benzo[b]thiophene-3-earboxaldehyde and derivatives.
Campaigne E and Neiss ES.
Journal of Heterocyclic Chemistry, 3(1), 46-50 (1966)
(Z)-2-(Benzo[b] thiophen-3-ylmethylene)-1-azabicyclo [2.2.2] octan-3-one.
Sonar VN, et al.
Acta Crystallographica Section E, Structure Reports Online, 59(11), o1726-o1728 (2003)
Synthesis and properties of isomerically pure anthrabisbenzothiophenes.
Lehnherr D, et al.
Organic Letters, 14(1), 62-65 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![Benzo[b]thiophene-2-carboxaldehyde 97%](/deepweb/assets/sigmaaldrich/product/structures/321/060/32405a4e-5720-4c6d-91cf-115c747270c4/640/32405a4e-5720-4c6d-91cf-115c747270c4.png)