466867
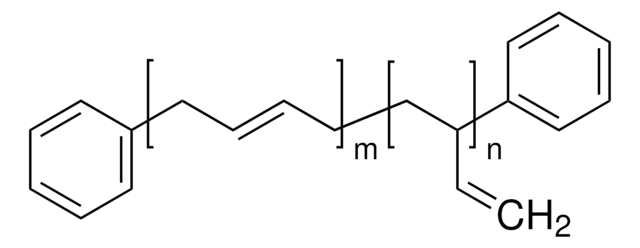
Polybutadiene, predominantly 1,2-addition
approx. 90% 1,2-vinyl
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
[CH2CH(CH=CH2)]n
CAS Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
refractive index
n20/D 1.5002 (lit.)
viscosity
30-100 poise(45 °C)(lit.)
transition temp
Tg −30 °C
density
0.86 g/mL at 25 °C (lit.)
SMILES string
C=CC=C
InChI
1S/C4H8/c1-3-4-2/h3-4H,1-2H3/b4-3+
InChI key
IAQRGUVFOMOMEM-ONEGZZNKSA-N
Application
- Transforming Polybutadiene with Tetrazine Click Chemistry into Antioxidant Foams That Fluoresce with Oxidation: This research uses tetrazine click chemistry to modify polybutadiene, enhancing its properties to create antioxidant foams that respond to oxidation with fluorescence, demonstrating innovative ways to utilize 1,2-addition polybutadiene (RE Bagge et al., 2017).
- Isoxazoline-based porous polymer for the highly effective adsorption of 2,4,6-trinitrotoluene (TNT): Catalyst-free click polymerization between an in situ generated nitrile oxide with polybutadiene. This article explores the enhancement of polybutadiene′s adsorptive properties for environmental cleanup applications, emphasizing how its structural characteristics affect performance (H Deng et al., 2020).
- Modification of polybutadiene with trifluoromethyl and clickable azide groups in one shot: This paper describes the simultaneous introduction of trifluoromethyl and azide functional groups into polybutadiene, showcasing the versatility of 1,2-addition polybutadiene in chemical modifications (S Wang et al., 2021).
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Deniz Turgut et al.
PloS one, 5(12), e15551-e15551 (2010-12-24)
In recent years, there is aroused interest in expressing complex systems as networks of interacting nodes. Using descriptors from graph theory, it has been possible to classify many diverse systems derived from social and physical sciences alike. In particular, folded
Alexandra Sperschneider et al.
ACS nano, 4(10), 5609-5616 (2010-09-17)
We report on the quasi in situ scanning force microscopy nanotomography which proved to be a key method to effectively obtain a three-dimensional (3D) microdomain structure of a complex ABC triblock morphology. As an example, we studied polybutadiene-block-poly(2-vinyl pyridine)-block-poly(tert-butyl methacrylate)
Mechanochemistry: Demonstrated leverage.
Roman Boulatov
Nature chemistry, 5(2), 84-86 (2013-01-25)
Aleer M Yol et al.
Journal of the American Society for Mass Spectrometry, 24(1), 74-82 (2012-11-30)
[M + Ag](+) ions from cyclic and linear polystyrenes and polybutadienes, formed by matrix-assisted laser desorption ionization (MALDI), give rise to significantly different fragmentation patterns in tandem mass spectrometry (MS(2)) experiments. In both cases, fragmentation starts with homolytic cleavage at
Jiayin Yuan et al.
Macromolecular rapid communications, 32(15), 1157-1162 (2011-06-29)
Commercially available 1,2-PB was transformed into a well-defined reactive intermediate by quantitative bromination. The brominated polymer was used as a polyfunctional macroinitiator for the cationic ring-opening polymerization of 2-ethyl-2-oxazoline to yield a water-soluble brush polymer. Nucleophilic substitution of bromide by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![Bis[3-(triethoxysilyl)propyl] tetrasulfide technical, ≥90% (NMR)](/deepweb/assets/sigmaaldrich/product/structures/242/790/625f5cba-32bd-4acf-a3be-e119e9cf844f/640/625f5cba-32bd-4acf-a3be-e119e9cf844f.png)

