416916
Dicyclohexyliodoborane
97%
Synonym(s):
Dicyclohexylboryl iodide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(C6H11)2BI
CAS Number:
Molecular Weight:
304.02
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
reaction suitability
reagent type: reductant
bp
198-200 °C/1.25 mmHg (lit.)
density
1.325 g/mL at 25 °C (lit.)
SMILES string
IB(C1CCCCC1)C2CCCCC2
InChI
1S/C12H22BI/c14-13(11-7-3-1-4-8-11)12-9-5-2-6-10-12/h11-12H,1-10H2
InChI key
RWFGGTOYIFQXAO-UHFFFAOYSA-N
Application
Dicyclohexyliodoborane (Chx2BI) can be used as a reagent:
- For the enolboration of esters and tertiary amides to synthesize corresponding Z or E enolates.
- In the stereoselective preparation of β-hydroxy-α-trifluoromethyl carboxylic acids via haloborane-mediated diastereoselective aldol addition of aldehydes with trifluoropropanoic acid.
- In the total synthesis of pordamacrine A, and trocheliophorolide D.
Reactant for:
- Preparation of vinyloxyboranes
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A boron-based Ireland-Claisen approach to the synthesis of pordamacrine A
Seizert, Curtis A and Ferreira, Eric M
Tetrahedron, 73(29), 4186-4194 (2017)
Dicyclohexyliodoborane/Triethylamine-a new reagent which achieves the facile enolboration of esters and tertiary amides
Brown HC and Ganesan K
Tetrahedron Letters, 33(24), 3421-3424 (1992)
Enolboration. 6. Dicyclohexyliodoborane, a Versatile Reagent for the Stereoselective Synthesis of Either Z or E Enolates from Representative Esters
Ganesan K and Brown HC
The Journal of Organic Chemistry, 59(9), 2336-2340 (1994)
Total Synthesis of the Proposed Structure of Trocheliophorolide D
Hwang S, et al.
European Journal of Organic Chemistry, 2011(36), 7414-7418 (2011)
Diastereoselective synthesis of anti-3-hydroxy-2-trifluoromethyl carboxylic acids
Ramachandran PV, et al.
Tetrahedron Letters, 56(23), 3019-3022 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service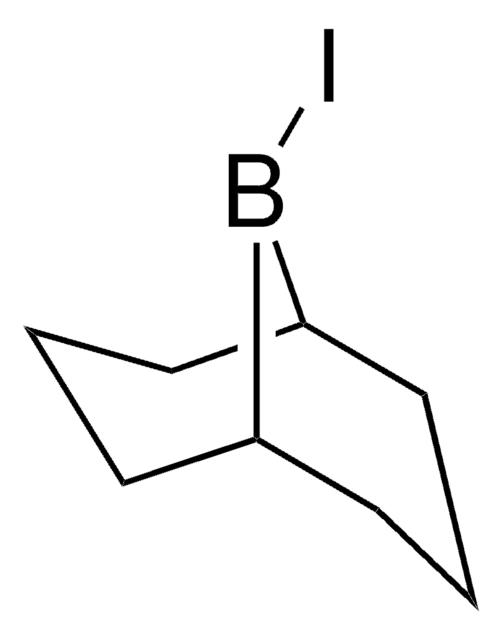
![9-Borabicyclo[3.3.1]nonane solution 0.5 M in THF](/deepweb/assets/sigmaaldrich/product/structures/180/891/8b64e597-269d-4780-98b6-40889dfd06b9/640/8b64e597-269d-4780-98b6-40889dfd06b9.png)



![9-Borabicyclo[3.3.1]nonane dimer](/deepweb/assets/sigmaaldrich/product/structures/203/431/624973a6-aec1-4b23-b6c4-013285ac418c/640/624973a6-aec1-4b23-b6c4-013285ac418c.png)




