407852
tert-Butyldimethylsilyl cyanide
97%
Synonym(s):
tert-Butyl-cyano-dimethylsilane
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)3CSi(CH3)2CN
CAS Number:
Molecular Weight:
141.29
Beilstein:
2234709
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
76-79 °C (lit.)
SMILES string
CC(C)(C)[Si](C)(C)C#N
InChI
1S/C7H15NSi/c1-7(2,3)9(4,5)6-8/h1-5H3
InChI key
CWAKIXKDPQTVTA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
tert-Butyldimethylsilyl cyanide (TBDMSCN) is a bulkier trialkylsilylcyanide. It participates in the cyanosilylation of enantiopure 4-oxoazetidine-2-carbaldehydes. Addition of TBDMSCN to sterically hindered ketones in the presence of Lewis acid or base catalyst has been studied. ZnI2-catalyzed addition of TBDSCN to 2,2-dimethylcyclohexanone, 2,2,6-trimethylcyclohexanone and 2,2,6,6-tetramethylcyclohexanone affords protected cyanohydrins.
Application
tert-Butyldimethylsilyl cyanide may be used as reagent for the formation of β-isonitrile alcohols via epoxides.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
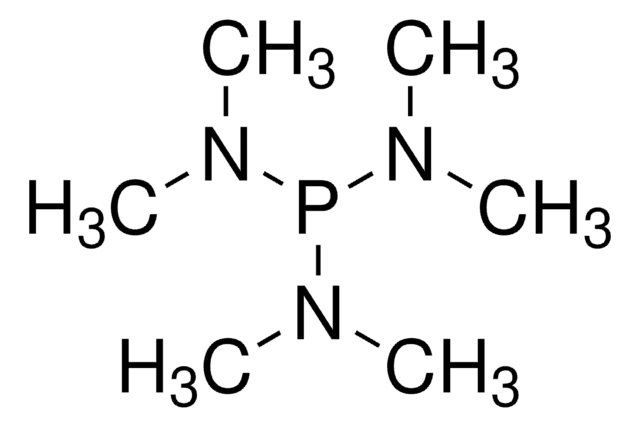
P(RNCH2CH2)N: efficient catalysts for the cyanosilylation of aldehydes and ketones.
Fetterly BM and Verkade JG.
Tetrahedron Letters, 46(46), 8061-8066 (2005)
The Journal of Organic Chemistry, 51, 5010-5010 (1986)
Benito Alcaide et al.
The Journal of organic chemistry, 72(21), 7980-7991 (2007-09-18)
The cyanosilylation of enantiopure 4-oxoazetidine-2-carbaldehydes with tert-butyldimethylsilyl cyanide was promoted by either molecular sieves or catalytic amount of sodium carbonate to give O-silylated beta-lactam cyanohydrins with good yield and diastereoselectivity. In contrast, Lewis acids did not effectively promote the cyanosilylation
Addition of tert-butyldimethyl-or tert-butyldiphenylsilyl cyanide to hindered ketones.
Golinski M, et al.
The Journal of Organic Chemistry, 58(1), 159-164 (1993)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service