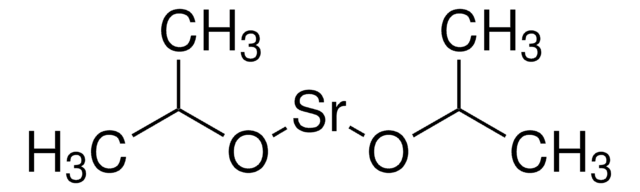388548
Strontium acetate
Synonym(s):
Strontium diacetate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3CO2)2Sr
CAS Number:
Molecular Weight:
205.71
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
impurities
~3% water
SMILES string
CC(=O)O[Sr]OC(C)=O
InChI
1S/2C2H4O2.Sr/c2*1-2(3)4;/h2*1H3,(H,3,4);/q;;+2/p-2
InChI key
RXSHXLOMRZJCLB-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Strontium acetate can be used as a strontium precursor to synthesize:
- Strontium titanate (SrTiO3) via hydrothermal synthesis with titanium isopropoxide.
- Lead strontium titanate thin films, applicable for the development of some tunable dielectric devices.
- Strontium cyanamide polymorphs via ammonia nitridation.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ammonia nitridation synthesis and structural change of strontium cyanamide polymorphs
Takeda T, et al.
J. Ceram. Soc. Jpn., 115(1347), 729-731 (2007)
A facile hydrothermal synthesis of SrTiO3 for dye sensitized solar cell application
Jayabal P, et al.
Journal of alloys and compounds, 586, 456-461 (2014)
D Cummins
The Journal of clinical dentistry, 22(4), 97-99 (2011-01-01)
This paper briefly discusses recent scientific and clinical research validating the effectiveness of a toothpaste containing 8.0% arginine and calcium carbonate, known as Pro-Argin technology, including clinical evidence for the superior efficacy of this toothpaste versus a potassium-based desensitizing toothpaste.
Charles R Parkinson et al.
The Journal of clinical dentistry, 22(3), 74-81 (2011-09-13)
The objective of this study was to evaluate the relative level of dentin tubule occlusion and dentin mineralization conferred by a 5% w/w calcium sodium phosphosilicate (45S5)/1450 ppm fluoride toothpaste in comparison to a range of commercial toothpastes reported to
N X West et al.
Journal of clinical periodontology, 24(4), 209-215 (1997-04-01)
A considerable number of varied agents are apparently effective in the treatment of dentine hypersensitivity. In particular, the literature supports the efficacy of strontium, potassium and fluoride containing toothpastes. This study was a double-blind, randomised, parallel group comparison of three
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service