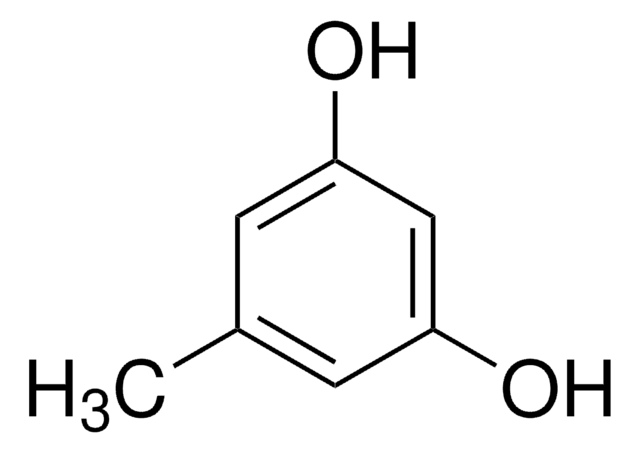
232505
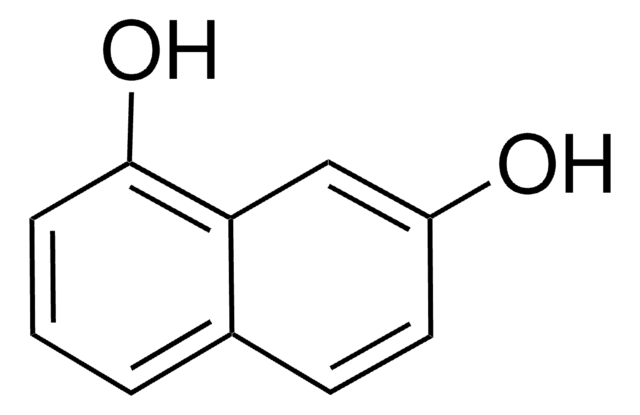
1,2-Dihydroxynaphthalene
technical grade
Synonym(s):
1,2-Naphthalenediol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C10H6(OH)2
CAS Number:
Molecular Weight:
160.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
mp
101-103 °C (lit.)
SMILES string
Oc1ccc2ccccc2c1O
InChI
1S/C10H8O2/c11-9-6-5-7-3-1-2-4-8(7)10(9)12/h1-6,11-12H
InChI key
NXPPAOGUKPJVDI-UHFFFAOYSA-N
General description
1,2-Dihydroxynaphthalene is the most sensitive biomarker for an internal exposure to naphthalene in humans.
Application
1,2-Dihydroxynaphthalene was used as substrate to investigate the rate of oxygen consumption by washed cell suspensions of Corynebacterium sp. strain C125 grown on o-xylene, tetralin or succinate.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Huilan Fu et al.
Molecular plant pathology, 21(10), 1337-1352 (2020-08-11)
The basal transcription factor II H (TFIIH) is a multicomponent complex. In the present study, we characterized a TFIIH subunit Tfb5 by analysing loss- and gain-of-function mutants to gain a better understanding of the molecular mechanisms underlying stress resistance and
Vandana P Swetha et al.
Applied and environmental microbiology, 71(10), 5951-5956 (2005-10-06)
Pseudomonas sp. strains C4, C5, and C6 utilize carbaryl as the sole source of carbon and energy. Identification of 1-naphthol, salicylate, and gentisate in the spent media; whole-cell O2 uptake on 1-naphthol, 1,2-dihydroxynaphthalene, salicylaldehyde, salicylate, and gentisate; and detection of
Katrina L Bosward et al.
Journal of dairy science, 99(3), 2142-2150 (2016-01-19)
Streptococcus agalactiae is a well-characterized bovine mastitis pathogen that is known to be highly contagious and capable of spreading rapidly in affected dairy herds. Loop-mediated isothermal amplification (LAMP) is a novel molecular diagnostic method that has the capability to provide
Muhammad Saeed et al.
Chemico-biological interactions, 165(3), 175-188 (2007-01-17)
Naphthalene is considered by the US Environmental Protection Agency to be a carcinogenic compound based on inhalation studies in rats. The primary metabolite of naphthalene is naphthalene 1,2-arene oxide. This unstable intermediate can lead to formation of 1-naphthol and naphthalene-1,2-dihydrodiol.
Sayamon Hongjaisee et al.
International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases, 102, 440-445 (2020-11-02)
The aim was to develop a reverse transcription loop-mediated isothermal amplification (RT-LAMP) assay for the detection of hepatitis C virus (HCV) in a single closed tube. Plasma samples were collected from 200 HCV-infected patients. HCV-RNA was detected by one-step RT-LAMP
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service