All Photos(1)
About This Item
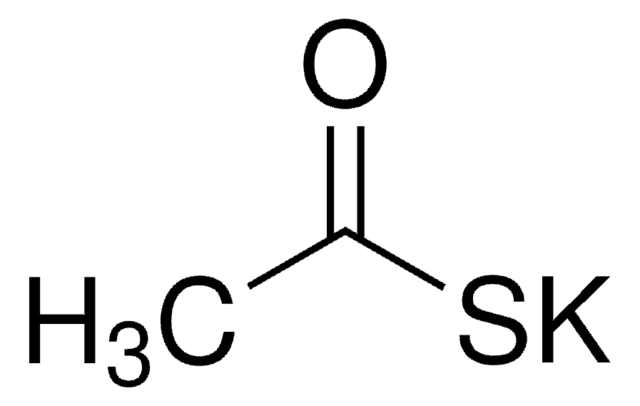
Linear Formula:
CF3COSC2H5
CAS Number:
Molecular Weight:
158.14
Beilstein:
1761564
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.376 (lit.)
bp
90.5 °C (lit.)
density
1.234 g/mL at 25 °C (lit.)
SMILES string
CCSC(=O)C(F)(F)F
InChI
1S/C4H5F3OS/c1-2-9-3(8)4(5,6)7/h2H2,1H3
InChI key
VGGUKFAVHPGNBF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
S-Ethyl trifluorothioacetate was used to selectively trifluoroacetylate amino groups in proteins.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
68.0 °F - closed cup
Flash Point(C)
20 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
V D Mehta et al.
Bioconjugate chemistry, 5(3), 257-261 (1994-05-01)
Fluorinated proteins have been synthesized and characterized as potential in vivo 19F magnetic resonance imaging (MRI) and spectroscopy (MRS) agents. Proteins labeled with fluorine include bovine serum albumin, gamma-globulin, and purified immunoglobulin (IgG). The amino groups in proteins were selectively
S Doonan et al.
The Italian journal of biochemistry, 28(6), 441-455 (1979-11-01)
Results obtained as part of a study of the primary structure of mitochondrial aspartate aminotransferase from pig heart are described. In particular, the S-aminoethylated protein was digested with trypsin and with the lysine specific protease from A. mellea. In the
María Eliana Defonsi Lestard et al.
The Journal of chemical physics, 131(21), 214303-214303 (2009-12-09)
The molecular structure and conformational properties of S-ethyl trifluorothioacetate, CF(3)COSCH(2)CH(3), were determined in the gas phase by electron diffraction and vibrational spectroscopy (IR and Raman). The experimental investigations were supplemented by ab initio (Moller Plesset of second order) and density
M Kamo et al.
European journal of biochemistry, 255(1), 162-171 (1998-08-06)
A vapor of S-ethyltrifluorothioacetate was found to specifically cleave the amino side of serine and threonine peptide bonds. The cleavage reactions were carried out at 50 degrees C for 6 h-24 h or at 30 degrees C for 24 h.
K L Hastings et al.
Immunopharmacology and immunotoxicology, 17(1), 201-213 (1995-02-01)
Halothane hepatitis appears to result from an inappropriate immune response to the products of halothane metabolism. Attempts to produce an animal model for halothane hepatitis have been largely unsuccessful. Although guinea pigs produce neoantigens following treatment with halothane, the subsequent
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service