161195
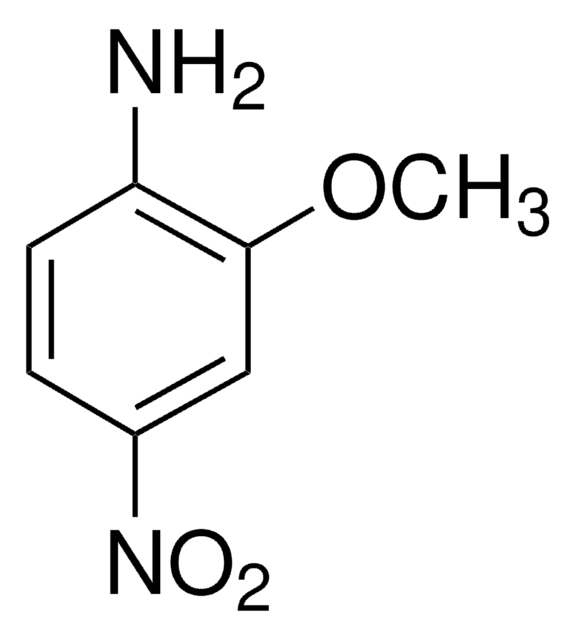
2-Methoxy-5-nitroaniline
98%
Synonym(s):
5-Nitro-o-anisidine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3OC6H3(NO2)NH2
CAS Number:
Molecular Weight:
168.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
117-119 °C (lit.)
SMILES string
COc1ccc(cc1N)[N+]([O-])=O
InChI
1S/C7H8N2O3/c1-12-7-3-2-5(9(10)11)4-6(7)8/h2-4H,8H2,1H3
InChI key
NIPDVSLAMPAWTP-UHFFFAOYSA-N
General description
2-Methoxy-5-nitroaniline is an aromatic metabolite of 2,4-dinitroanisole.
Application
2-Methoxy-5-nitroaniline was used in the synthesis of 5-(9-acridinylamino)-p-anisidines via reaction with 9-anilinoacridines. It was also used in the synthesis of disazo disperse dyes containing nitro and methoxy groups, used for the dyeing of polyester fibre.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
246.2 °F - closed cup
Flash Point(C)
119 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jidong Liang et al.
Journal of hazardous materials, 262, 281-287 (2013-09-18)
2,4-Dinitroanisole (DNAN) is an insensitive munitions compound considered to replace conventional explosives such as 2,4,6-trinitrotoluene (TNT). DNAN undergoes facile microbial reduction to 2-methoxy-5-nitroaniline (MENA) and 2,4-diaminoanisole (DAAN). This study investigated the inhibitory effect of DNAN, MENA, and DAAN toward various
Disperse dyes derived from 2-methoxy-5-nitroaniline.
Otutu JO and Osabohien E.
Orient. J. Chem., 25(4), 863-863 (2009)
A Dewanji et al.
Biometrics, 49(2), 367-377 (1993-06-01)
In this paper, a new method of estimating tumorigenic potency is proposed that takes into account information on survival and, when available, the underlying cause of death. Specifically, Weibull distributions are used to describe the time to tumor occurrence (X)
Valeriy A Bacherikov et al.
Bioorganic & medicinal chemistry, 13(23), 6513-6520 (2005-09-06)
A series of 5-(9-acridinylamino)anisidines were synthesized by condensing methoxy-substituted 1,3-phenylenediamines (10 and 11) with 9-chloroacridine derivatives to form 5-(9-acridinylamino)-m-anisidines (AMAs, 14a-e) and 5-(9-acridinylamino)-o-anisidines (AOAs, 15a-e). 5-(9-Acridinylamino)-p-anisidines (APAs, 17a-e) were synthesized by reacting 2-methoxy-5-nitroaniline (12) with 9-anilinoacridines, followed by reduction. The
5-Nitro-ortho-anisidine.
IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans, 27, 133-139 (1982-04-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service