Recommended Products
Quality Level
product line
Novabiochem®
form
beads
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
manufacturer/tradename
Novabiochem®
application(s)
peptide synthesis
functional group
amine
storage temp.
2-8°C
General description
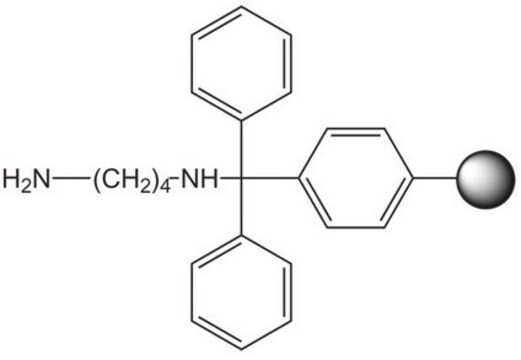
An acid-labile resin for the preparation of N-acyl or N-alkyl cysteamines. The free amino functionality of the resin-bound cysteamine can be readily acylated or reductively alkylated using standard procedures. Cleavage can be effected with electrophilic oxidants such as I2 or Tl(3) to produce a dimeric disulfide bridged product, or with 50-100% TFA to give the monomeric sulfhydryl product. Peptidylaminoethylthiols produced in this manner have been used to prepare PEG-conjugates by chemoselective ligation to pegylated maleimide [1]. This method is particularly useful for forming intramolecular disulfide bridges in molecules containing two thiol groups where one is protected with Acm. For a similar application, see [2,3,4].
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references
[1] J. Zhang, et al. Poster 162 presented at the 16 American Peptide Symposium, Minneapolis, 1999.
[2] A. v. Vliet, et al. in ′Innovation & Perspectives in Solid Phase Synthesis, 2nd International Symposium′, R. Epton (Eds), Intercept UK Ltd., Andover, 1992, pp. 475.
[3] A. v. Vliet, et al. in ′Peptides 1992, Proc.22nd European Peptide Symposium′, C. H. Schneider & A. N. Eberle (Eds), ESCOM, Leiden, 1993, pp. 279.
[4] A. v. Vliet, et al. in ′Peptides, Chemistry, Structure, &Biology, Proc. 13th American Peptide Symposium′, R. S. Hodges & J. A. Smith (Eds), ESCOM, Leiden, 1994, pp. 151.
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references
[1] J. Zhang, et al. Poster 162 presented at the 16 American Peptide Symposium, Minneapolis, 1999.
[2] A. v. Vliet, et al. in ′Innovation & Perspectives in Solid Phase Synthesis, 2nd International Symposium′, R. Epton (Eds), Intercept UK Ltd., Andover, 1992, pp. 475.
[3] A. v. Vliet, et al. in ′Peptides 1992, Proc.22nd European Peptide Symposium′, C. H. Schneider & A. N. Eberle (Eds), ESCOM, Leiden, 1993, pp. 279.
[4] A. v. Vliet, et al. in ′Peptides, Chemistry, Structure, &Biology, Proc. 13th American Peptide Symposium′, R. S. Hodges & J. A. Smith (Eds), ESCOM, Leiden, 1994, pp. 151.
Linkage
Replaces: 01-64-0107
Analysis Note
Color (visual): white to yellow to beige
Appearance of substance (visual): beads
Loading (determined from the substitution of the Fmoc-Leu loaded resin): 1.00 - 2.00 mmol/g
Swelling Volume (in DMF): lot specific result
The polymer matrix is copoly (styrene-1% DVB), 200 - 400 mesh.
Appearance of substance (visual): beads
Loading (determined from the substitution of the Fmoc-Leu loaded resin): 1.00 - 2.00 mmol/g
Swelling Volume (in DMF): lot specific result
The polymer matrix is copoly (styrene-1% DVB), 200 - 400 mesh.
Legal Information
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service