M13807
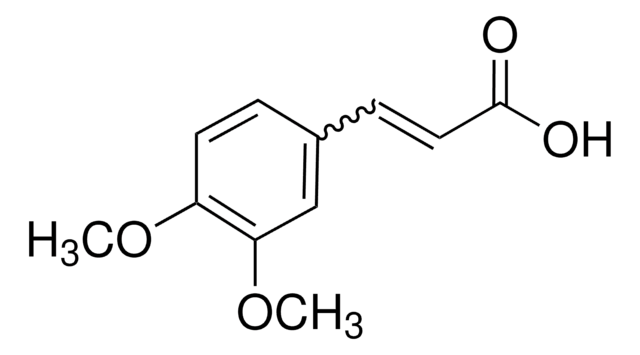
4-Methoxycinnamic acid, predominantly trans
99%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3OC6H4CH=CHCO2H
CAS Number:
Molecular Weight:
178.18
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
99%
form
liquid crystal
mp
170-173 °C (lit.)
transition temp
crystalline phase to nematic phase 173.5 °C
nematic phase to isotropic phase 190 °C
SMILES string
COc1ccc(\C=C\C(O)=O)cc1
InChI
1S/C10H10O3/c1-13-9-5-2-8(3-6-9)4-7-10(11)12/h2-7H,1H3,(H,11,12)/b7-4+
InChI key
AFDXODALSZRGIH-QPJJXVBHSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yoshio Nakagawa et al.
Biochemical pharmacology, 66(1), 63-73 (2003-06-24)
The metabolism and action of trans-anethole (anethole) and the estrogen-like activity of the compound and its metabolites were studied in freshly isolated rat hepatocytes and cultured MCF-7 human breast cancer cells, respectively. The incubation of hepatocytes with anethole (0.25-2.0mM) caused
S Adisakwattana et al.
Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme, 43(11), 766-773 (2011-10-20)
p-Methoxycinnamic acid (p-MCA) is a cinnamic acid derivative that shows various pharmacologic actions such as hepatoprotective and antihyperglycemic activities. The present study was to elucidate the mechanisms by which p-MCA increases [Ca²⁺]i and insulin secretion in INS-1 cells. p-MCA (100
Natasha Cook et al.
The Australasian journal of dermatology, 43(2), 133-135 (2002-05-02)
We report a case of photoallergic contact dermatitis to two sunscreen agents, methoxycinnamate and oxybenzone, occurring in a 6-year-old Asian boy.
Sirintorn Yibchok-anun et al.
Basic & clinical pharmacology & toxicology, 102(5), 476-482 (2008-03-19)
This study investigated the effect of p-methoxycinnamic acid (p-MCA) on plasma glucose and insulin concentrations in normal and streptozotocin-induced diabetic rats. In both fasting and glucose-loading conditions, an oral administration of p-MCA (40-100 mg/kg) significantly decreased plasma glucose and also
H V Meyers et al.
Carbohydrate research, 197, 15-32 (1990-03-25)
This study completes the spectroscopic basis for a novel oligosaccharide microanalytical method, wherein a derivatization sequence provides monosaccharide subunits bearing two types of exciton-coupling chromophore groups ("bichromophoric") for circular dichroic spectroscopy, namely 4-bromobenzoate (lambda max 245 nm) and 4-methoxycinnamate (lambda
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service