All Photos(1)
About This Item
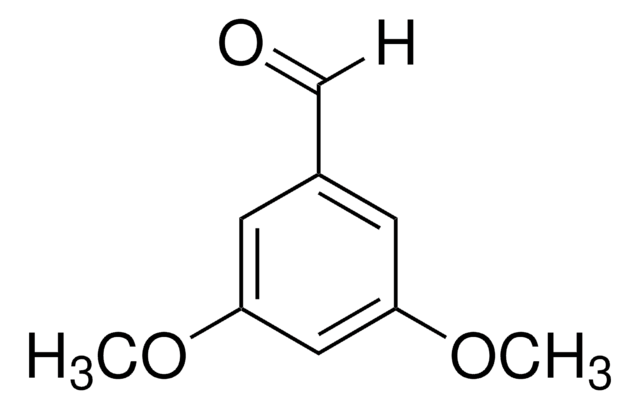
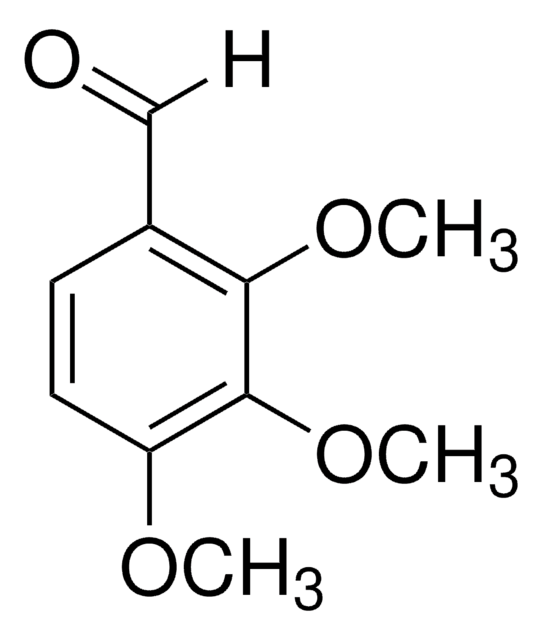
Linear Formula:
(CH3O)2C6H3CHO
CAS Number:
Molecular Weight:
166.17
Beilstein:
607989
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
crystals
bp
165 °C/10 mmHg (lit.)
mp
67-69 °C (lit.)
SMILES string
COc1ccc(C=O)c(OC)c1
InChI
1S/C9H10O3/c1-11-8-4-3-7(6-10)9(5-8)12-2/h3-6H,1-2H3
InChI key
LWRSYTXEQUUTKW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Outi Keinänen et al.
Nuclear medicine and biology, 67, 27-35 (2018-11-01)
18F-fluoroglycosylation via oxime formation is a chemoselective and mild radiolabeling method for sensitive molecules. Glycosylation can also improve the bioavailability, in vivo kinetics, and stability of the compound in blood, as well as accelerate clearance of biomolecules. A typical synthesis
Anne M Vissers et al.
Phytochemical analysis : PCA, 28(6), 487-495 (2017-06-15)
Phlorotannins are complex mixtures of phloroglucinol oligomers connected via C-C (fucols) or C-O-C (phlorethols) linkages. Their uniformity in subunits and large molecular weight hamper their structural analysis. Despite its commercial relevance for alginate extraction, phlorotannins in Laminaria digitata have not
Justin Maresh et al.
Bioorganic & medicinal chemistry letters, 17(12), 3281-3286 (2007-05-02)
VirA-VirG two-component system regulates the vir (virulence) operon in response to specific host factors (xenognosins) in the plant pathogen Agrobacterium tumefaciens. Using whole cell assays, stable inhibitors inspired by the labile natural benzoxazinone inhibitor HDMBOA are developed. It is found
Marcelo D Catarino et al.
Marine drugs, 17(3) (2019-03-13)
Phlorotannins are phloroglucinol-based phenolic compounds, occurring particularly in brown macroalgae, that have been recognized for their promising bioactive properties. In this study, the extraction of phlorotannins from Fucus vesiculosus was evaluated with particular emphasis on the influential parameters, including the
Ulrik Boas et al.
Journal of combinatorial chemistry, 4(3), 223-228 (2002-05-15)
The tris(alkoxy)benzyl backbone amide linker (BAL) has found widespread application in solid-phase synthesis. The key intermediate for preparation of para BAL (p-BAL) is 2,6-dimethoxy-4-hydroxybenzaldehyde; several reports on its synthesis have appeared. However, the ortho analogue of the handle (o-BAL) has
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service