91917
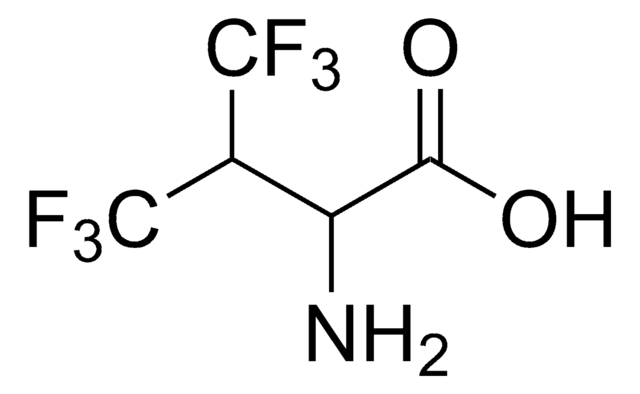
5,5,5-Trifluoro-DL-leucine
≥98.0% (sum of isomers, HPLC)
Synonym(s):
(±)-2-Amino-4-(trifluoromethyl)pentanoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H10F3NO2
CAS Number:
Molecular Weight:
185.14
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (sum of isomers, HPLC)
form
crystals
reaction suitability
reaction type: solution phase peptide synthesis
application(s)
peptide synthesis
SMILES string
CC(CC(N)C(O)=O)C(F)(F)F
InChI
1S/C6H10F3NO2/c1-3(6(7,8)9)2-4(10)5(11)12/h3-4H,2,10H2,1H3,(H,11,12)
InChI key
XFGVJLGVINCWDP-UHFFFAOYSA-N
General description
5,5,5-Trifluoro-DL-leucine, also known as trifluoroleucine, is an analog of L-leucine amino acid, often used to synthesize highly fluorinated peptides.
Application
5,5,5-trifluoroleucine can be incorporated into the coiled-coil peptides to increase their thermal stability.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
E Casalone et al.
Yeast (Chichester, England), 16(6), 539-545 (2000-05-03)
The function of the open reading frame (ORF) YOR108w of Saccharomyces cerevisiae has been analysed. The deletion of this ORF from chromosome XV did not give an identifiable phenotype. A mutant in which both ORF YOR108w and LEU4 gene have
D I Johnson et al.
Journal of bacteriology, 155(1), 49-55 (1983-07-01)
Mutants of Escherichia coli K-12 resistant to either the threonine analog DL-alpha-amino-beta-hydroxyvaleric acid or the leucine analog 5',5',5'-trifluoro-DL-leucine were isolated. One DL-alpha-amino-beta-hydroxyvaleric acid-resistant mutant strain, designated SP572, constitutively expressed the thr and ilv operons. The mutant allele, avr-16, was localized
Stabilization of coiled-coil peptide domains by introduction of trifluoroleucine
Y Tang
Biochemistry, 40, 2790-2796 (2001)
I Bardazzi et al.
Folia microbiologica, 49(5), 534-538 (2005-02-11)
Two new plasmids, pEC3 and pECkan, were constructed and their use in yeast transformation described. Both plasmids are derivative of the pRS416 vector, in which the URA3 auxotrophic marker was replaced by the LEU4* gene (pEC3) or the kanMX4 gene
A coiled coil with a fluorous core
B Bilgicer
Journal of the American Chemical Society, 19, 4393-4399 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service