907278
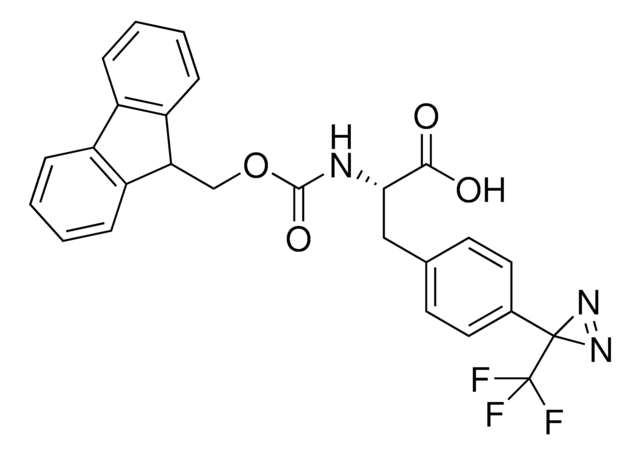
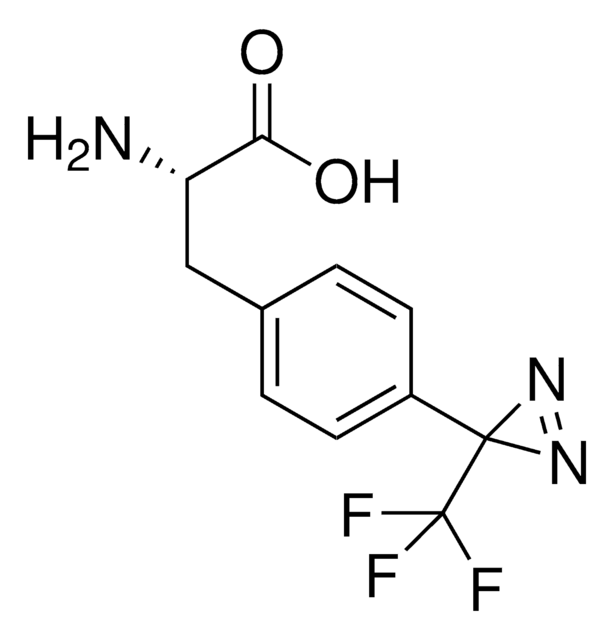
H-L-Photo-leucine HCl
≥98%
Synonym(s):
(S)-2-Amino-3-(3-methyl-3H-diazirin-3-yl)propanoic acid hydrochloride, (S)-2-Amino-3-(3H-diazirin-3-yl)butanoic acid hydrochloride, Diazirine amino acid, Photo-Leu, Photo-crosslinking amino acid, Photoprobe building block
About This Item
Recommended Products
Assay
≥98%
form
solid
reaction suitability
reaction type: solution phase peptide synthesis
availability
available only in USA
application(s)
peptide synthesis
storage temp.
−20°C
Application
Other Notes
for generation of homogeneous conjugates from wild-type antibodies
Mechanistic studies of a small-molecule modulator of SMN2 splicing
Protein-Polymer Conjugation via Ligand Affinity and Photoactivation of Glutathione S-Transferase
Direct Interaction between an Allosteric Agonist Pepducin and the Chemokine Receptor CXCR4
Photo-leucine and photo-methionine allow identification of protein-?protein interactions in living cells
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Self-react. C
Storage Class Code
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 3
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service