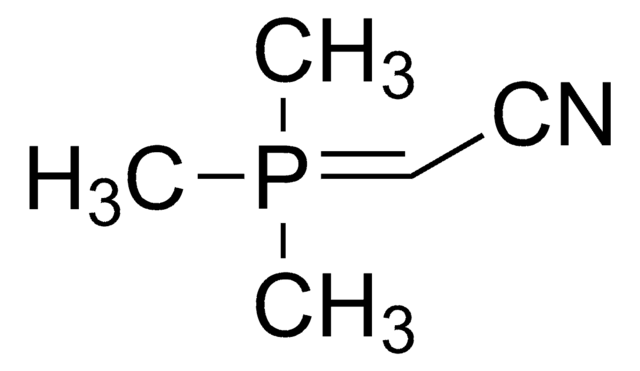
761060
(Tributylphosphoranylidene)acetonitrile
97%
Synonym(s):
(Cyanomethylene)tributylphosphorane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C14H28NP
CAS Number:
Molecular Weight:
241.35
UNSPSC Code:
12352000
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.500 (lit.)
density
0.921 g/mL at 25 °C
storage temp.
2-8°C
InChI
1S/C14H28NP/c1-4-7-11-16(14-10-15,12-8-5-2)13-9-6-3/h14H,4-9,11-13H2,1-3H3
InChI key
OZMLUMPWPFZWTP-UHFFFAOYSA-N
General description
(Tributylphosphoranylidene)acetonitrile or cyanomethylenetrimethylphosphorane (Bu3P=CHCN) is a Wittig reagent employed in the transformation of the carbonyl compounds, including aldehydes, esters, and lactones into the corresponding unsaturated nitriles.
Application
(Tributylphosphoranylidene)acetonitrile can be utilized as a reagent in the:
- Stereoselective synthesis of skytanthine and other O- and N-containing heterocycles by Mitsunobu intramolecular cycloalkylation.
- Wittig olefination of esters, lactones, N-Boc lactam, and cyclic imide to corresponding Wittig products.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
(Tributylphosphoranylidene) acetonitrile
Wyatt, Peter B
Encyclopedia of Reagents for Organic Synthesis, Second Edition (2001)
Formation of heterocycles by the Mitsunobu reaction. Stereoselective synthesis of (+)-α-skytanthine
Tsunoda T, et al.
Tetrahedron Letters, 37(14), 2463-2466 (1996)
Cyanomethylenetrimethylphosphorane, a powerful reagent for the Wittig olefination of esters, lactones and imides
Tsunoda T, et al.
Tetrahedron Letters, 41(2), 235-237 (2000)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service






![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)