55083
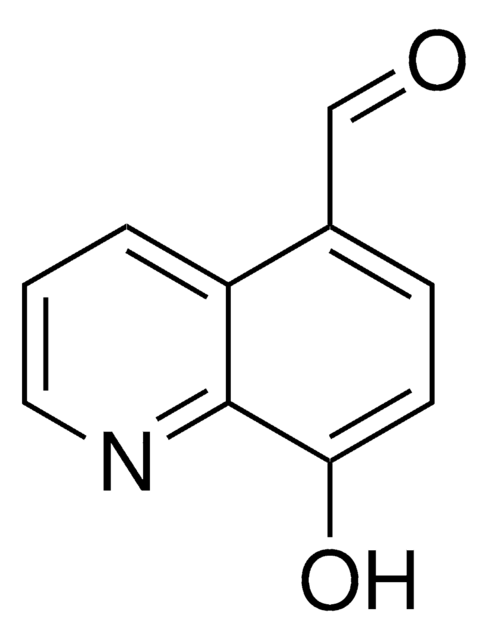
8-Hydroxy-2-quinolinecarboxaldehyde
≥98.0% (GC)
Synonym(s):
2-Formyl-8-hydroxyquinoline, 2-Formyl-8-quinolinol, 8-Hydroxyquinoline-2-aldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H7NO2
CAS Number:
Molecular Weight:
173.17
Beilstein:
127519
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (GC)
form
solid
mp
97-100 °C
functional group
aldehyde
SMILES string
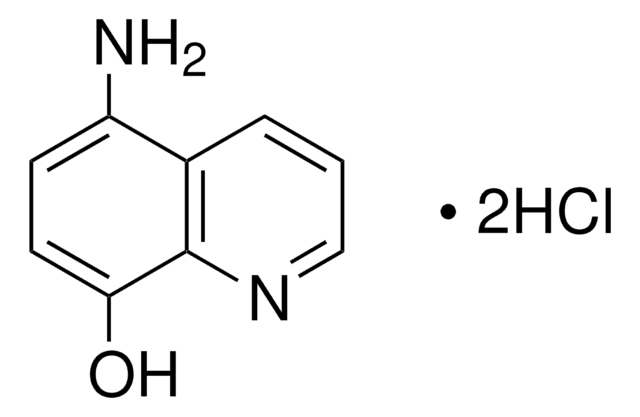
Oc1cccc2ccc(C=O)nc12
InChI
1S/C10H7NO2/c12-6-8-5-4-7-2-1-3-9(13)10(7)11-8/h1-6,13H
InChI key
SLBPIHCMXPQAIQ-UHFFFAOYSA-N
General description
8-Hydroxy-2-quinolinecarboxaldehyde can be prepared from 2-methylquinolin-8-ol via oxidation using selenium dioxide.
Application
8-Hydroxy-2-quinolinecarboxaldehyde (8-hydroxyquinoline-2-carbaldehyde) may be used in the preparation of:
- 8-hydroxy-2-quinoline-1-aminopyrene by Schiff-base reaction with 1-aminopyrene
- (E)-2-((2-(pyridin-2-yl)hydrazono)methyl)quinolin-8-ol by coupling with 2-hydrazinopyridine
- 8-hydroxyquinoline-2-carbaldehyde oxime
- 2-[(8-Hydroxyquinoline)-2-methylaminoethyl]-β-D-glucopyranoside
Other Notes
Building block for the synthesis of complexing agents
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
S. Sugata et al.
Chemical & Pharmaceutical Bulletin, 35, 2623-2623 (1987)
Saptarshi Ghosh et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 188, 252-257 (2017-07-21)
The present work reports detailed photophysics of a coumarin based Schiff base, namely, (E)-7-(((8-hydroxyquinolin-2-yl)methylene)amino)-4-methyl-2H-chromen-2-one (HMC) in different solvents of varying polarity exploiting steady state absorption, fluorescence and time resolved fluorescence spectroscopy. The dominant photophysical features of HMC are discussed in
Rhombus-shaped tetranuclear [Ln4] complexes [Ln= Dy (III) and Ho (III)]: synthesis, structure, and SMM behavior.
Chandrasekhar V, et al.
Inorganic Chemistry, 52(11), 6346-6353 (2013)
Nadia Alessandra Carmo Dos Santos et al.
Dalton transactions (Cambridge, England : 2003), 46(47), 16455-16464 (2017-11-17)
Novel cobalt, nickel, and iron complexes based on the pentadentate 8-hydroxyquinoline-di(2-picolyl)amine ligand were synthesized and thoroughly characterized. X-ray structures of both the cobalt and iron complexes were also obtained, showing the tendency to adopt a pseudo-octahedral geometry by coordination of
Soluble sugar-based quinoline derivatives as new antioxidant modulators of metal-induced amyloid aggregation.
Oliveri V, et al.
Inorganic Chemistry, 54(6), 2591-2602 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service