All Photos(1)
About This Item
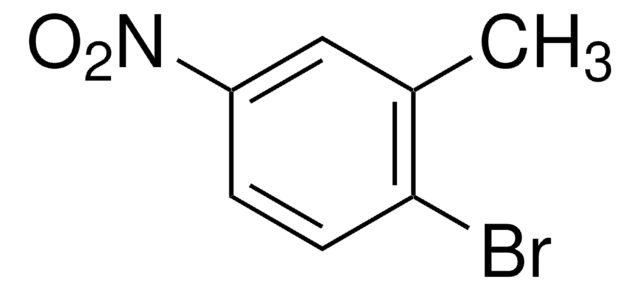
Linear Formula:
CH3C6H3(NO2)Br
CAS Number:
Molecular Weight:
216.03
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
bp
130 °C/12 mmHg (lit.)
mp
45-48 °C (lit.)
solubility
water: insoluble(lit.)
functional group
bromo
nitro
SMILES string
Cc1ccc(Br)cc1[N+]([O-])=O
InChI
1S/C7H6BrNO2/c1-5-2-3-6(8)4-7(5)9(10)11/h2-4H,1H3
InChI key
KZNXALJXBRSMFL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4-Bromo-2-nitrotoluene is a nitrotoluene derivative. It can be synthesized by the regioselective bromination of o-nitrotoluene.
Application
4-Bromo-2-nitrotoluene may be used as a starting material in the synthesis of the following:
- 4-bromo-2-nitrobenzylidene
- 4-bromo-2-nitrobenzaldehyde
- 4-bromo-2-chlorotoluene
- 4-bromo-2-nitrobenzoic acid by oxidation
- 6-bromoindole by Batcho-Leimgruber indole synthesis
- 3-(4-bromo-2-nitrophenyl)-2-[2-(tert-butyldimethylsilanyloxy)ethyl]-N-(2,4-dichloro-6-iodophenyl)-N-methoxymethylacrylamide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The bromo-2-nitrobenzoic acids.
Erickson JLE, et al.
Journal of the American Chemical Society, 74(22), 5621-5623 (1952)
A Simple, Safe and Efficient Synthesis of Tyrian Purple (6, 6'-Dibromoindigo).
Wolk JL and Frimer AA.
Molecules (Basel), 15(8), 5561-5580 (2010)
Olga V Barykina et al.
Organic letters, 12(11), 2664-2667 (2010-05-08)
The synthesis of (+/-)-eusynstyelamide A has been accomplished in six steps in 13% overall yield from 6-bromoindole, methyl glycidate, and Boc-protected agmatine. If oxygen is carefully excluded from the reaction, the key NaOH-catalyzed aldol dimerization of the alpha-ketoamide proceeded efficiently
Synthesis of 4-Bromo-2-chlorotoluene.
Xue XM, et al.
Chinese Journal of Pharmaceuticals / Chung-kuo i yao kung yeh tsa chih, 37(9), 588-588 (2006)
Jae Hong Seo et al.
The Journal of organic chemistry, 71(23), 8891-8900 (2006-11-04)
An efficient synthetic strategy for installation of the two vicinal quaternary carbon centers of the communesins is reported. Key steps include the O-allylation/Claisen rearrangement of spirolactone systems, which are formed by tandem intramolecular Heck cyclization/carbonylation. Substituent and solvent effects on
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service







![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
