357987
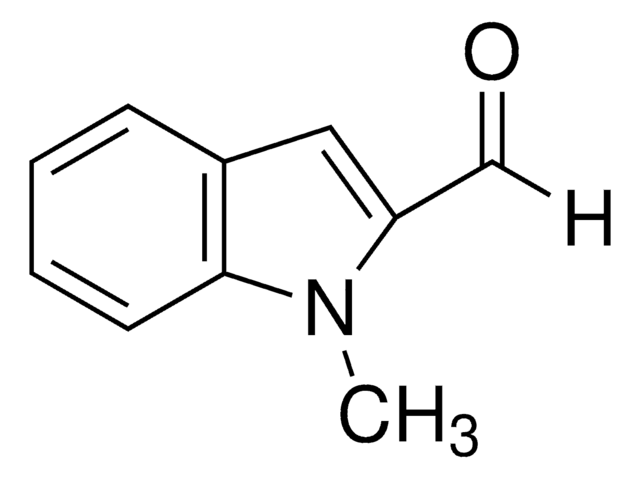
1-Methylindole-3-carboxaldehyde
97%
Synonym(s):
3-Formyl-1-methylindole, NSC 83042
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H9NO
CAS Number:
Molecular Weight:
159.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
70-72 °C (lit.)
functional group
aldehyde
SMILES string
Cn1cc(C=O)c2ccccc12
InChI
1S/C10H9NO/c1-11-6-8(7-12)9-4-2-3-5-10(9)11/h2-7H,1H3
InChI key
KXYBYRKRRGSZCX-UHFFFAOYSA-N
General description
1-Methylindole-3-carboxaldehyde is a heterocyclic indole aldehyde. 1-Methylindole-3-carboxaldehyde on condensation with 2-hydroxybenzohydrazide yields Schiff base.
Application
1-Methylindole-3-carboxaldehyde may be used in the synthesis of (Z)-3-(1-methyl-1H-indol-3-yl)-2-(thiophen-3-yl)acrylonitrile, via base-catalyzed condensation with thiophene-3-acetonitrile. It was also used in the preparation of monomer, required for the synthesis of poly(3-vinyl-1-methylindole).
- Reactant for preparation of nitroolefins and β-nitroalcohols via microwave- or ultrasound-assisted Henry reactions
- Reactant for synthesis of quinolinones via three-component Ugi reaction
- Reactant for synthesis of α-ketoamides as inhibitors of Dengue virus protease with antiviral activity in cell-culture
- Reactant for preparation of thiazolopyrimidinones as inhibitors of Bcl-2 proteins
- Reactant for preparation of vinylindoles via Peterson olefination or olefination with Nysted reagent
- Reactant for preparation of indolyl alkenes from microwave-enhanced Knoevenagel condensation as antibacterial agents
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Wagee A Yehye et al.
Acta crystallographica. Section E, Structure reports online, 64(Pt 9), o1824-o1824 (2008-01-01)
In the crystal structure of the title Schiff-base, C(20)H(21)N(3)O(4), the amino group forms an N-H⋯O hydrogen bond to the acetyl group of an adjacent mol-ecule, forming a zigzag chain. The 2-hydr-oxy group is inter-nally hydrogen bonded to the amido group
Preliminary analysis of the 1H-and 13C-NMR spectra of poly (3-vinyl-1-methylindole).
Trumbo DL.
Polymer Bull., 37(1), 75-80 (1996)
Vijayakumar N Sonar et al.
Acta crystallographica. Section C, Crystal structure communications, 60(Pt 3), o217-o218 (2004-03-09)
The title compound, C16H12N2S, has been synthesized by base-catalyzed condensation of 1-methylindole-3-carboxaldehyde with thiophene-3-acetonitrile. The product assumes an approximately planar Z configuration. The molecule has a thienyl-ring flip disorder.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service