About This Item
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.502 (lit.)
bp
82-84 °C/15 mmHg (lit.)
mp
15-17 °C (lit.)
density
1.317 g/mL at 25 °C (lit.)
storage temp.
2-8°C
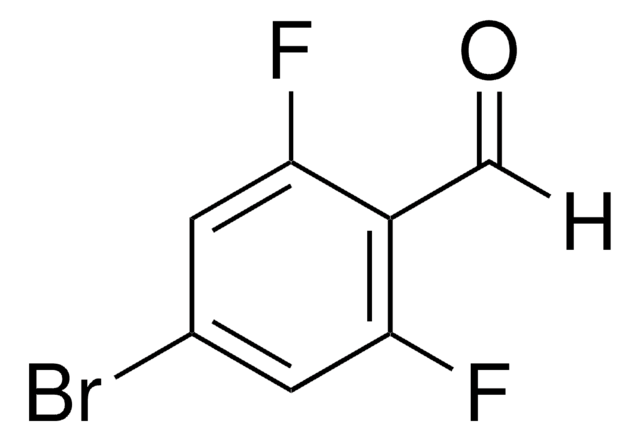
SMILES string
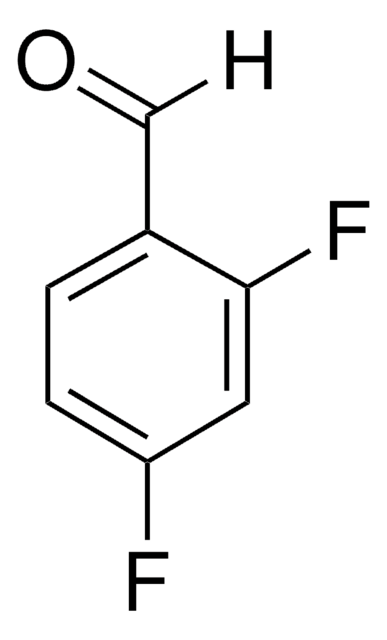
Fc1cccc(F)c1C=O
InChI
1S/C7H4F2O/c8-6-2-1-3-7(9)5(6)4-10/h1-4H
InChI key
SOWRUJSGHKNOKN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- 5-Cyano-6-(2,6-difluorophenyl)-5,6-dihydro-2-thiouracil via one-pot cyclocondensation reaction with ethyl cyanoacetate and thiourea.
- 1-(2,6-Difluorobenzyl)-2-(2,6-difluorophenyl)-benzimidazole by reacting with 1,2-phenylenediamine in the presence of a catalytic amount of p-toluenesulfonic acid.
- (3E)-4-(2,6-Difluorophenyl)-3-buten-2-one by Wittig olefination reaction with acetylmethylidenetriphenyl phosphorane.
- Methyl 4-fluorobenzo[b]thiophene-2-carboxylate by treating with methyl thioglycolate in the presence of K2CO3.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1C
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 2
Flash Point(F)
180.5 °F - closed cup
Flash Point(C)
82.5 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service