All Photos(1)
About This Item
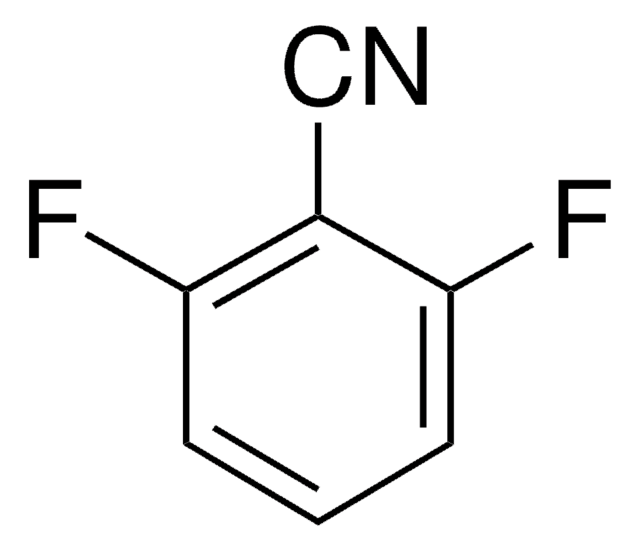
Linear Formula:
F2C6H3CN
CAS Number:
Molecular Weight:
139.10
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
52-54 °C (lit.)
functional group
fluoro
SMILES string
Fc1ccc(cc1F)C#N
InChI
1S/C7H3F2N/c8-6-2-1-5(4-10)3-7(6)9/h1-3H
InChI key
BTBFCBQZFMQBNT-UHFFFAOYSA-N
Application
3,4-Difluorobenzonitrile has been used in the preparation of:
- fluorophenoxy herbicides
- fluorine substituted benzyl amides
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
156.2 °F - closed cup
Flash Point(C)
69 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Rajendra P Tangallapally et al.
Journal of medicinal chemistry, 48(26), 8261-8269 (2005-12-22)
In an ongoing effort to develop new and potent antituberculosis agents, a second-generation series of nitrofuranyl amides was synthesized on the basis of the lead compound 5-nitrofuran-2-carboxylic acid 3,4-dimethoxybenzylamide. The primary design consideration was to improve the solubility and consequently
Synthesis of 3, 4-difluorobenzonitrile and monofluorobenzonitriles by means of halogen-exchange fluorination.
Suzuki H and Kimura Y.
Journal of Fluorine Chemistry, 52(3), 341-351 (1991)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service