258822
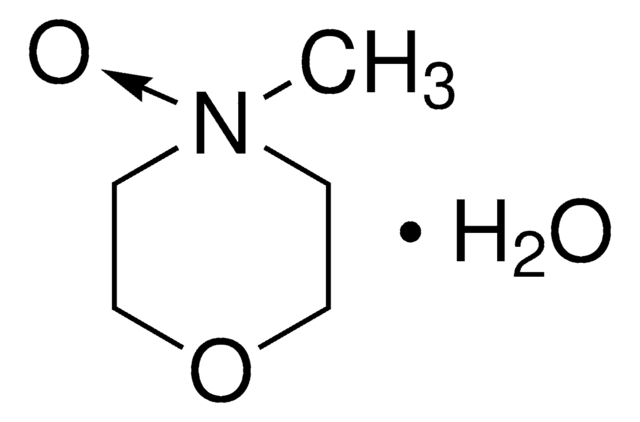
4-Methylmorpholine N-oxide solution
50 wt. % in H2O
Synonym(s):
NMMO solution, NMO solution, NSC 73198, NSC 82153
About This Item
Recommended Products
form
liquid
Quality Level
reaction suitability
reagent type: oxidant
concentration
50 wt. % in H2O
refractive index
n20/D 1.4201
pH
9.00 ( in neat)
bp
118.5 °C
mp
−20 °C
density
1.13 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
C[N+]1([O-])CCOCC1
InChI
1S/C5H11NO2/c1-6(7)2-4-8-5-3-6/h2-5H2,1H3
InChI key
LFTLOKWAGJYHHR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- 4-Methylmorpholine N-oxide (NMO) is widely used as a co-oxidant to regenerate osmium tetroxide (OsO4) catalyst during dihydroxylation of alkenes.
- In the presence of catalytic amounts of tetra-n-propylammonium perruthenate (TPAP), NMO oxidizes secondary amines to the corresponding imines.
- 4-Methylmorpholine N-oxide solution can be used to oxidize activated primary halides to aldehydes and secondary halides to ketones, respectively.
- It can also be used to promote stereoselective intermolecular Pauson-Khand reaction for the synthesis of cyclopentenones.
Reagent or Reactant for:
- Cyclocondensation and cyclization in the enantioselective synthesis of oxazolomycin A
- Wharton rearrangement and stereoselective dihydroxylation reactions
- Reduction of amine N-oxides by diboron reagents
- Synthesis of polysaccharide blend fibers
- Synthesis of cellulose / modified nano-SiO2 composite packaging films
- Copper-catalyzed oxidative coupling for preparation of propargylamines
Used as a pretreatment for techno-economical study of ethanol and biogas production from spruce wood
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Repr. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service