244465
1-Octyne
97%
Synonym(s):
1-Ethynylhexane, Hexylacetylene, n-Hexylacetylene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
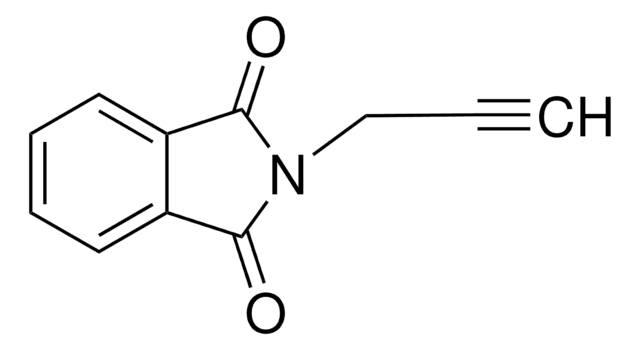
CH3(CH2)5C≡CH
CAS Number:
Molecular Weight:
110.20
Beilstein:
1734494
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39010411
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
37.7 mmHg ( 37.7 °C)
Quality Level
Assay
97%
form
liquid
impurities
≤3% 1-bromohexane
refractive index
n20/D 1.416 (lit.)
bp
127-128 °C (lit.)
mp
−80 °C (lit.)
density
0.747 g/mL at 25 °C (lit.)
SMILES string
CCCCCCC#C
InChI
1S/C8H14/c1-3-5-7-8-6-4-2/h1H,4-8H2,2H3
InChI key
UMIPWJGWASORKV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1-Octyne was used as a mechanism-based inhibitor of AlkB (nonheme di-iron alkane monooxygenase).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
60.8 °F - closed cup
Flash Point(C)
16 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
D L Kline et al.
Medical and veterinary entomology, 21(4), 323-331 (2007-12-21)
Field studies were conducted at wooded wetlands in Gainesville, FL, U.S.A., to assess responses of natural populations of adult mosquitoes (Diptera: Culicidae) to American Biophysics MM-X and Coleman MD-2500 traps baited with enantiomers of 1-octen-3-ol, a naturally occurring compound, and
Ishwar Singh et al.
Organic & biomolecular chemistry, 10(33), 6633-6639 (2012-07-04)
Strain promoted cycloaddition is presented as a tool for RNA conjugation on the solid phase; RNA-cyclooctyne conjugates are prepared by cycloaddition to both azide (strain-promoted azide-alkyne cycloaddition, SPAAC) and nitrile oxide dipoles (strain-promoted nitrile oxide-alkyne cycloaddition, SPNOAC). The conjugation is
I N White et al.
The Biochemical journal, 220(1), 85-94 (1984-05-15)
[1,2-14C]Oct-l-yne was used to investigate metabolic activation of the ethynyl substituent in vitro. Activation of octyne by liver microsomal cytochrome P-450-dependent enzymes gave intermediate(s) that bound covalently to protein, DNA and to haem. The time course and extent of covalent
Hernan Alonso et al.
Applied and environmental microbiology, 78(22), 7946-7953 (2012-09-04)
The alkane hydroxylase system of Pseudomonas putida GPo1 allows it to use alkanes as the sole source of carbon and energy. Bacterial alkane hydroxylases have tremendous potential as biocatalysts for the stereo- and regioselective transformation of a wide range of
Jean-Charles Bruyere et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 25(34), 8061-8069 (2019-03-26)
The reactivity of ZnII dialkyl species ZnMe2 with a cyclic(alkyl)(amino)carbene, 1-[2,6-bis(1-methylethyl)phenyl]-3,3,5,5-tetramethyl-2-pyrrolidinylidene (CAAC, 1), was studied and extended to the preparation of robust CAAC-supported ZnII Lewis acidic organocations. CAAC adduct of ZnMe2 (2), formed from a 1:1 mixture of 1 and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service