G6649
Genistein
≥98% (HPLC), powder, tyrosine protein kinase inhibitor
Synonym(s):
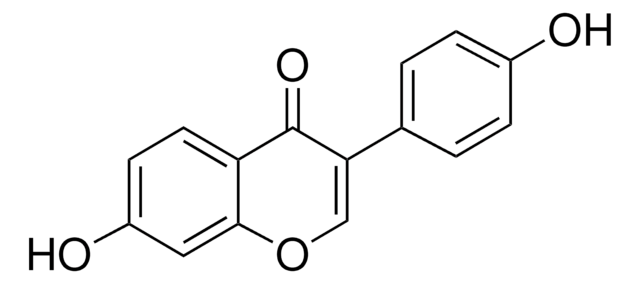
4′,5,7-Trihydroxyisoflavone, 5,7-Dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one
About This Item
Recommended Products
product name
Genistein, synthetic, ≥98% (HPLC), powder
biological source
synthetic
Quality Level
Assay
≥98% (HPLC)
form
powder
color
off-white to yellow
mp
297.0-298.0 °C
solubility
DMSO: soluble
ethanol: soluble
storage temp.
−20°C
SMILES string
Oc1ccc(cc1)C2=COc3cc(O)cc(O)c3C2=O
InChI
1S/C15H10O5/c16-9-3-1-8(2-4-9)11-7-20-13-6-10(17)5-12(18)14(13)15(11)19/h1-7,16-18H
InChI key
TZBJGXHYKVUXJN-UHFFFAOYSA-N
Gene Information
human ... AKT1(207) , CYP19A1(1588) , EGFR(1956) , ESR1(2099) , ESR2(2100)
mouse ... Esr1(13982) , Hexa(15211)
rat ... Adora1(29290) , Adora2a(25369) , Ar(24208)
Looking for similar products? Visit Product Comparison Guide
General description
Application
- as a test compound to access its estrogeniic acivity
- as an oestrogenic ligand to carry out identical reporter gene activation assays and also used to examine the binding of genistein to hERβ
- as an endocytosis inhibitor to test the possible effect of endocytosed advanced glycation end - bovine serum albumin (AGE-BSA) on lysosomes
Biochem/physiol Actions
Features and Benefits
Preparation Note
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Chronic inflammation is an underlying factor in the development and progression of many of the chronic diseases of aging, such as arthritis, atherosclerosis, diabetes, and cancer.
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Discover Bioactive Small Molecules for Kinase Phosphatase Biology
Discover Bioactive Small Molecules for Kinase Phosphatase Biology
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service