P3502
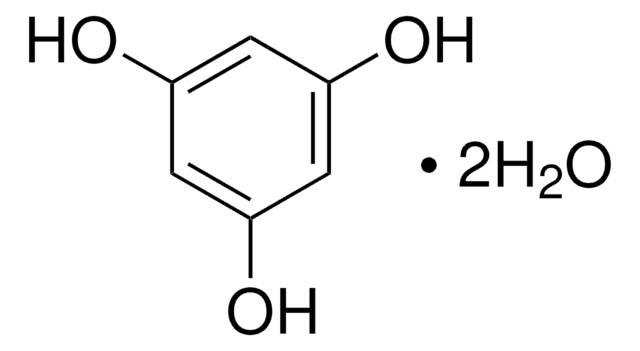
Phloroglucinol
Used to detect the presence of wood fiber.
Synonym(s):
1,3,5-Trihydroxybenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H6O3
CAS Number:
Molecular Weight:
126.11
Beilstein:
1341907
EC Number:
MDL number:
UNSPSC Code:
12171500
PubChem Substance ID:
NACRES:
NA.47
Recommended Products
Quality Level
form
powder or crystals
mp
215-220 °C
solubility
ethanol: 50 mg/mL (clear to hazy)
application(s)
diagnostic assay manufacturing
hematology
histology
storage temp.
room temp
SMILES string
Oc1cc(O)cc(O)c1
InChI
1S/C6H6O3/c7-4-1-5(8)3-6(9)2-4/h1-3,7-9H
InChI key
QCDYQQDYXPDABM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Phloroglucinol (phlo) is a phenol derivative that shows a cytoprotective effect from oxidative damage by enhancing the activity of cellular catalase. It can also be used to prepare synthetic analogs of A-type proanthocyanidins (PACs) such as 2,8-dioxabicyclo[3.3.1]nonane derivatives by reacting with the corresponding flavylium salts. Phlo can react with benzaldehyde derivatives to form phloroglucinol-based microporous polymeric organic frameworks (phlo-POF) with potential applications in ion exchange and gas adsorption.
Phloroglucinol is an antispasmodic and a derivative of phenol.
Application
Phloroglucinol has been used to prevent hyperhydricity by promoting the lignification of shoots. It has also been used in PG (phloroglucinol)-sucrose treatments for the growth and development of in vitro derived shoot tips. Phloroglucinol has been used to stain root sections. It has also been used to stain plant sections to identify plant materials like lignin.
Phloroglucinol has been used to stain root sections. It has also been used to stain plant sections to identify plant materials like lignin.
Biochem/physiol Actions
Phloroglucinol displays antispasmodic activity. It is a trihydroxybenzene with antithrombotic, profibrinolytic, antimicrobial, antimalarial, cancer chemopreventive, anti-HIV, and anti-leishmanial activities. Phloroglucinol (PG) is a biosynthetic precursor of the 2,4-diacetylphloroglucinol (DAPG) an antibiotic against soil-borne diseases. Phloroglucinol is a useful intermediate because it is polyfunctional.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Poplar stems show opposite epigenetic patterns during winter
dormancy and vegetative growth
dormancy and vegetative growth
Daniel Conde
Trees (Berlin, Germany : West) (2013)
Component traits of plant water use are modulated by vapour
pressure deficit in pearl millet (Pennisetum glaucum (L.) R.Br.)
pressure deficit in pearl millet (Pennisetum glaucum (L.) R.Br.)
Jana Kholova
Functional Ecology (2016)
Role of antispasmodics in the treatment of irritable bowel syndrome
Anita Annahazi
World Journal of Gastrointestinal Oncology (2014)
Reactive Oxygen Species and Cellular Interactions
Between Mycosphaerella fijiensis and Banana
Between Mycosphaerella fijiensis and Banana
Maria
Tropical Plant Biology (2011)
Yong Li et al.
Bioorganic & medicinal chemistry, 17(5), 1963-1973 (2009-02-10)
Seven phlorotannins were isolated and characterized from an edible marine brown alga Ecklonia cava (EC), along with three common sterol derivatives (fucosterol, ergosterol, and cholesterol) according to the comprehensive spectral analysis of MS and NMR data. Compounds 5 (7-phloro eckol)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service