14335
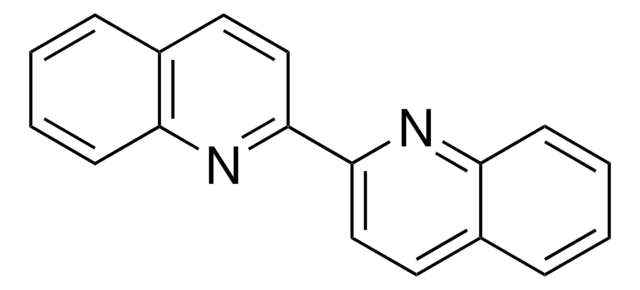
2,2′-Biquinoline-4,4′-dicarboxylic acid
≥90% (TLC)
Synonym(s):
2,2′-Bicinchoninic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C20H12N2O4
CAS Number:
Molecular Weight:
344.32
Beilstein:
321561
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Quality Level
Assay
≥90% (TLC)
form
powder
storage temp.
2-8°C
SMILES string
OC(=O)c1cc(nc2ccccc12)-c3cc(C(O)=O)c4ccccc4n3
InChI
1S/C20H12N2O4/c23-19(24)13-9-17(21-15-7-3-1-5-11(13)15)18-10-14(20(25)26)12-6-2-4-8-16(12)22-18/h1-10H,(H,23,24)(H,25,26)
InChI key
AFYNADDZULBEJA-UHFFFAOYSA-N
Application
2,2′-Biquinoline-4,4′-dicarboxylic acid has been used in a study that synthesized and structurally characterized six metal-organic coordination polymers.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Geeta S Paranjape et al.
Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology, 8(1), 312-322 (2012-12-18)
Soluble aggregated forms of amyloid-β protein (Aβ) have garnered significant attention recently for their role in Alzheimer's disease (AD). Protofibrils are a subset of these soluble species and are considered intermediates in the aggregation pathway to mature Aβ fibrils. Biological
Anna Mero et al.
Methods in molecular biology (Clifton, N.J.), 751, 95-129 (2011-06-16)
PEGylation, the covalent linking of PEG chains, has become the leading drug delivery approach for proteins. This technique initiated its first steps almost 40 years ago, and since then, a variety of methods and strategies for protein-polymer coupling have been
Sandra W Chan et al.
Experimental neurology, 238(1), 29-37 (2011-10-18)
Levodopa (L-DOPA), a close structural analogue of the protein amino acid L-tyrosine, can substitute for L-tyrosine in protein synthesis and be mistakenly incorporated into newly synthesised proteins in vitro. We show that L-DOPA-containing proteins are present in the brain in
The effect of organic acid on self-assembly process: Syntheses and characterizations of six novel cadmium(II)/zinc(II) complexes derived from mixed ligands
Li, N., et al.
Inorgorganica Chimica Acta, 362, 3475-3483 (2009)
Chandra K Dixit et al.
The Analyst, 136(7), 1406-1411 (2011-01-27)
The non-specific loss of protein analytes can have a major effect on assay results particularly where the concentrations of such analytes are extremely low and the matrix is complex. This report assesses how the protein incubated in sample tubes may
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service