07260
6-Aminohexanoic acid
≥98.5% (NT)
Synonym(s):
6-Aminocaproic acid, 6-ACA, Aca, Acp, Aha, Ahx, acikaprin, afibrin, amicar, ε-Aminocaproic acid, 6-Aminohexanoic acid, EACA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
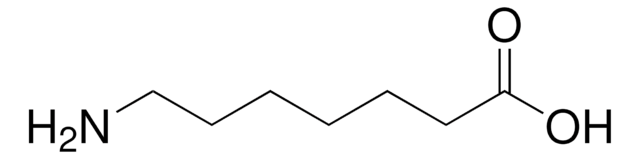
H2N(CH2)5CO2H
CAS Number:
Molecular Weight:
131.17
Beilstein:
906872
EC Number:
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥98.5% (NT)
mp
207-209 °C (dec.) (lit.)
SMILES string
NCCCCCC(O)=O
InChI
1S/C6H13NO2/c7-5-3-1-2-4-6(8)9/h1-5,7H2,(H,8,9)
InChI key
SLXKOJJOQWFEFD-UHFFFAOYSA-N
Gene Information
human ... PLAT(5327) , PLG(5340)
Looking for similar products? Visit Product Comparison Guide
General description
6-Aminohexanoic acid, or 6-aminocaproic acid, acts as an inhibitor of serine proteases. It shares structural similarities with the natural amino acid lysine but lacks an α-amino group.
Application
6-Aminohexanoic acid has been used as a component of the growth medium for tenogenic differentiation of mesenchymal stem cells. It has also been used in the preparation of modified graphene quantum dots.
Biochem/physiol Actions
Lysine analog. Promotes rapid dissociation of plasmin, thereby inhibiting the activation of plasminogen and subsequent fibrinolysis. Reported to inhibit plasminogen binding to activated platelets. An early report indicated that it inhibits the activation of the first component of the complement system. Binds and inactivates Carboxypeptidase B.
Other Notes
Improves solubilization of membrane proteins in electrophoresis
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
404.6 - 408.2 °F
Flash Point(C)
207 - 209 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Purification strategies for membrane proteins
VON JAGOW, et al.
Journal of Separation Science, 2, 1-8 (2003)
Kumiko Okazaki et al.
Plant physiology, 141(2), 546-556 (2006-04-11)
Most extant cyanobacteria contain C16 fatty acids in the sn-2 positions of glycerolipids, which are regulated by lysophosphatidic acid acyltransferase (LPAAT; EC 2.3.1.51). Synechocystis sp. PCC6803 contains sll1848, sll1752, and slr2060 as putative acyltransferase genes. sll1848 was recently reported to
The Importance of 6-Aminohexanoic Acid as a Hydrophobic, Flexible Structural Element
Agnieszka Markowska, et al.
International Journal of Molecular Sciences, 22(22), 12122-12122 (2021)
Post-synthetic modification of graphene quantum dots bestows enhanced biosensing and antibiofilm ability: efficiency face
Neha Agrawal, et.al
Royal Society of Chemistry Advances, 12(20), 12310-12320 (2022)
H Schägger et al.
Analytical biochemistry, 199(2), 223-231 (1991-12-01)
A discontinuous electrophoretic system for the isolation of membrane proteins from acrylamide gels has been developed using equipment for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Coomassie dyes were introduced to induce a charge shift on the proteins and aminocaproic acid
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service