R-011
Ritalinic acid hydrochloride solution
1.0 mg/mL in methanol (as free base), ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Recommended Products
grade
certified reference material
Quality Level
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
concentration
1.0 mg/mL in methanol (as free base)
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
forensics and toxicology
format
single component solution
storage temp.
−20°C
SMILES string
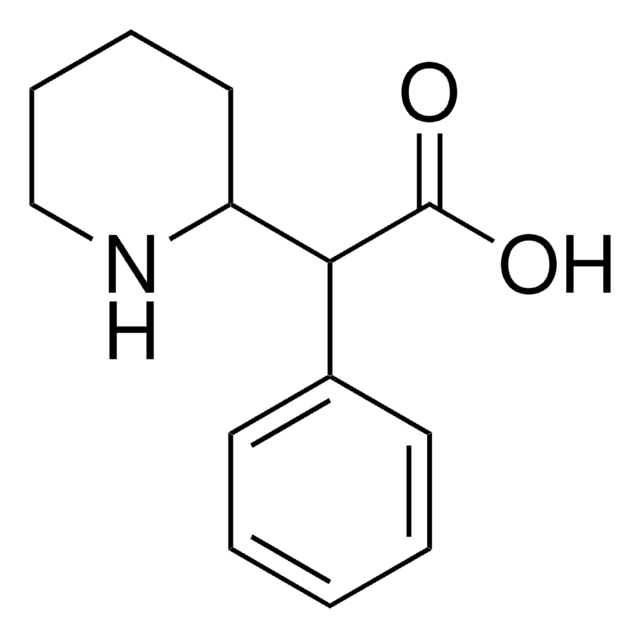
Cl.OC(=O)C(C1CCCCN1)c2ccccc2
InChI
1S/C13H17NO2.ClH/c15-13(16)12(10-6-2-1-3-7-10)11-8-4-5-9-14-11;/h1-3,6-7,11-12,14H,4-5,8-9H2,(H,15,16);1H
InChI key
SCUMDQFFZZGUQY-UHFFFAOYSA-N
General description
Application
- Genotoxicity research in non-human primates: Ritalinic acid hydrochloride, a primary metabolite of methylphenidate, is used in genetic toxicology studies to evaluate potential genotoxic effects, aiding in the assessment of safety profiles for neuropharmacological treatments (Morris et al., 2009).
- Pharmacokinetics and mutagenicity studies: Ritalinic acid hydrochloride is used in dose-range and mutagenicity research. It helps in studying the pharmacokinetics and toxicological impact of methylphenidate, offering important insights into its safety and effectiveness in therapeutic applications (Manjanatha et al., 2008).
- Bioequivalence and food effect studies: In clinical pharmacology, ritalinic acid hydrochloride is instrumental in conducting bioequivalence studies of dexmethylphenidate, examining the influence of food on drug absorption and metabolism, crucial for optimizing drug administration protocols (Teo et al., 2004).
- Childhood pharmacotherapy: Research using ritalinic acid hydrochloride explores the effects of methylphenidate when administered with or without food, highlighting its impact on the plasma concentration of the drug and its acid metabolite, informing pediatric dosing guidelines (Chan et al., 1983).
- Hyperkinetic disorders treatment studies: Ritalinic acid hydrochloride supports pharmacokinetic studies in children with hyperkinetic disorders, aiding in understanding how methylphenidate behaves within the body to improve therapeutic strategies (Hungund et al., 1979).
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Target Organs
Eyes,Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
49.5 °F - closed cup
Flash Point(C)
9.7 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service