All Photos(1)
About This Item
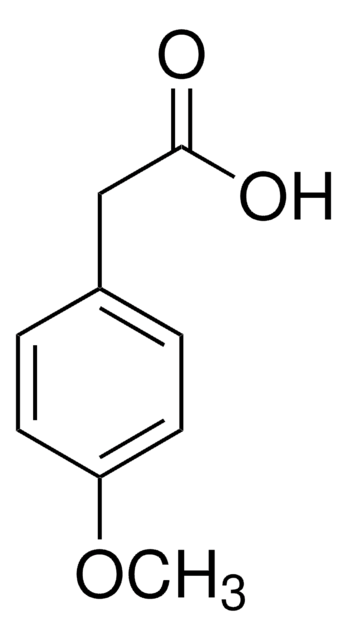
Linear Formula:
CH3OC6H4CH2CO2H
CAS Number:
Molecular Weight:
166.17
Beilstein:
2614004
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
mp
65-69 °C (lit.)
SMILES string
COc1cccc(CC(O)=O)c1
InChI
1S/C9H10O3/c1-12-8-4-2-3-7(5-8)6-9(10)11/h2-5H,6H2,1H3,(H,10,11)
InChI key
LEGPZHPSIPPYIO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ramisetti Nageswara Rao et al.
Chirality, 24(4), 339-344 (2012-02-22)
(R)-(-)-α-Methoxy phenyl acetic acid, (S)-(-)-1,1'-(2-naphthol), and (R)-(+)-α-methoxy-α-trifluoromethyl phenyl acetic acid were evaluated as chiral shift reagents (CSRs) for (1)H NMR spectroscopic resolution and determination of R and S enantiomers of modafinil (MDL) in bulk drugs and formulations. Effects of the
Bohang Zhou et al.
European journal of medicinal chemistry, 200, 112415-112415 (2020-05-27)
As simple analogues of the natural compound chelerythrine, a novel anti-cholinesterase 2-phenylisoquinolin-2-ium scaffold was designed by structure imitation. The activity evaluation led to the discovery of seven compounds with potent anti-acetylcholinesterase activity with IC50 values of ≤0.72 μM, superior to chelerythrine
Georgios I Panoutsopoulos et al.
Chemical research in toxicology, 17(10), 1368-1376 (2004-10-19)
Aliphatic aldehydes have a high affinity toward aldehyde dehydrogenase activity but are relatively poor substrates of aldehyde oxidase and xanthine oxidase. In addition, the oxidation of xenobiotic-derived aromatic aldehydes by the latter enzymes has not been studied to any great
M Chen et al.
Antimicrobial agents and chemotherapy, 45(7), 2023-2029 (2001-06-16)
Our previous studies have shown that chalcones exhibit potent antileishmanial and antimalarial activities in vitro and in vivo. Preliminary studies showed that these compounds destroyed the ultrastructure of Leishmania parasite mitochondria and inhibited the respiration and the activity of mitochondrial
Phytotoxins in Rhizoctonia solani: isolation and biological activity of m-hydroxy- and m-methoxyphenylacetic acids.
N B Mandava et al.
Journal of agricultural and food chemistry, 28(1), 71-75 (1980-01-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service