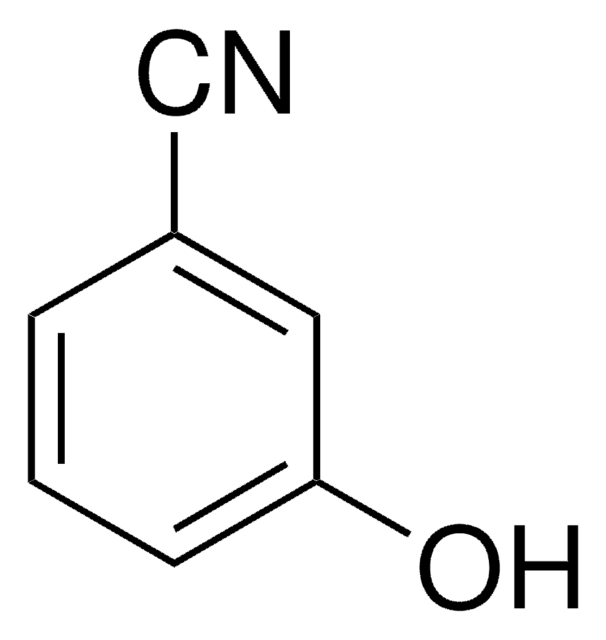
C94009
4-Cyanophenol
95%
Synonym(s):
4-Hydroxybenzonitrile
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
NCC6H4OH
CAS Number:
Molecular Weight:
119.12
Beilstein:
386130
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
crystals
mp
110-113 °C (lit.)
SMILES string
Oc1ccc(cc1)C#N
InChI
1S/C7H5NO/c8-5-6-1-3-7(9)4-2-6/h1-4,9H
InChI key
CVNOWLNNPYYEOH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
4-Cyanophenol is a precursor for the synthesis of a vasodilator, Levcromakalim. Bromination of 4-cyanophenol results in bromoxynil, a commercial herbicide. It can also be used as a component of deep eutectic solvent (DES) mixture.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
High atom efficient and environment-friendly preparation of herbicides bromoxynil and ioxynil.
Joshi G and Patil RD
Indian Journal of Chemistry, 49B, 1678-1680 (2010)
Nitrogen-doped carbons prepared from eutectic mixtures as metal-free oxygen reduction catalysts.
Lopez-Salas N, et al.
Journal of Material Chemistry A, 4(2), 478-488 (2016)
Synthesis and antihypertensive activity of 4-(cyclic amido)-2H-1-benzopyrans.
Ashwood VA, et al.
Journal of Medicinal Chemistry, 29(11), 2194-2201 (1986)
G D Touraille et al.
Environmental science & technology, 39(10), 3723-3731 (2005-06-16)
Despite the skin's excellent barrier function, dermal exposure to soil contaminated with toxic chemicals can represent a significant health hazard (e.g., via multiple work related contacts in the farming and waste disposal industries). The development of environmental standards or limits
D B Harper
The International journal of biochemistry, 17(6), 677-683 (1985-01-01)
The purification and properties of an enzyme from Nocardia sp. which catalyses the conversion of p-hydroxybenzonitrile to p-hydroxybenzoic acid and ammonia without intermediate formation of the amide is described. The enzyme displayed a broad pH optimum between 7.0 and 9.5
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service