764213
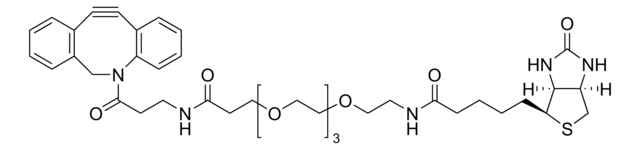
Biotin-PEG4-alkyne
for copper catalyzed click labeling
Synonym(s):
Polyethylene glycol, Acetylene-PEG4-biotin conjugate
About This Item
Recommended Products
Assay
95%
form
solid
reaction suitability
reaction type: click chemistry
mp
55-64 °C
storage temp.
−20°C
SMILES string
O=C(NCCOCCOCCOCCOCC#C)CCCC[C@@H](SC1)[C@@]2([H])[C@]1([H])NC(N2)=O
InChI
1S/C21H35N3O6S/c1-2-8-27-10-12-29-14-15-30-13-11-28-9-7-22-19(25)6-4-3-5-18-20-17(16-31-18)23-21(26)24-20/h1,17-18,20H,3-16H2,(H,22,25)(H2,23,24,26)/t17-,18-,20-/m1/s1
InChI key
SKMJWNZZFUDLKQ-QWFCFKBJSA-N
Related Categories
Application
Automate your Biotin tagging with Synple Automated Synthesis Platform (SYNPLE-SC002)
Other Notes
Fluorescent Heterotelechelic Single-Chain Polymer Nanoparticles: Synthesis, Spectroscopy, and Cellular Imaging
Arginine-Selective Chemical Labeling Approach for Identification and Enrichment of Reactive Arginine Residues in Proteins
Selective Imaging of Gram-Negative and Gram-Positive Microbiotas in the Mouse Gut
Metabolic Oligosaccharide Engineering with Alkyne Sialic Acids Confers Neuraminidase Resistance and Inhibits Influenza Reproduction
A Modular Probe Strategy for Drug Localization, Target Identification and Target Occupancy Measurement on Single Cell Level
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine 97%](/deepweb/assets/sigmaaldrich/product/structures/179/695/86a721c8-2a4c-4e4f-bc36-6276ce7a941f/640/86a721c8-2a4c-4e4f-bc36-6276ce7a941f.png)