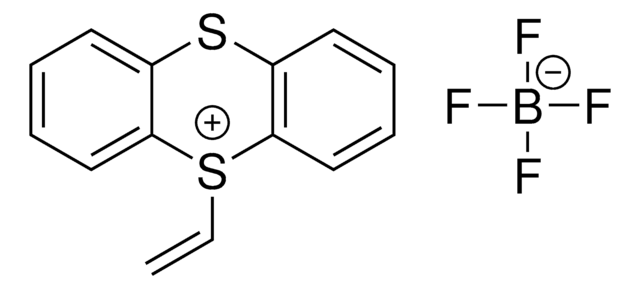
695637
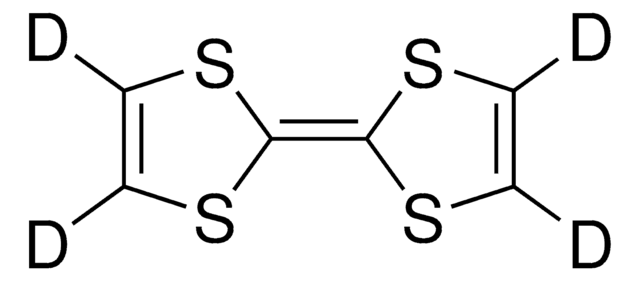
Dibenzotetrathiafulvalene
97%
Synonym(s):
DBTTF, [2,2’]Bi[benzo[1,3]dithiolylidene]
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C14H8S4
CAS Number:
Molecular Weight:
304.47
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
97%
form
solid
mp
239-243 °C
SMILES string
S1C(\Sc2ccccc12)=C3/Sc4ccccc4S3
InChI
1S/C14H8S4/c1-2-6-10-9(5-1)15-13(16-10)14-17-11-7-3-4-8-12(11)18-14/h1-8H
InChI key
OVIRUXIWCFZJQC-UHFFFAOYSA-N
General description
Dibenzotetrathiafulvalene (DBTTF) is an organic semiconductor that is completely conjugated with a symmetric structure. It forms stacks of planar molecules with a distance of 3.948 Å. DBTTF has a high mobility of charges and can be prepared from anthranilic acid and purified by sublimating in vacuum.
Application
DB-TTF is used to make semiconducting charge transfer salts with electron accepting (n-type) materials, for example TCNQ (Aldrich Prod. No. 157635) and F4TCNQ (Aldrich Prod. No. 376779 ).
DBTTF is an organoelectronic material that forms a charge transferring complex with a variety of semiconducting crystals which include 7,7,8,8-tetracyanoquinodimethane (TCNQ) and 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ). These materials can be in the form of single crystals and thin films for the fabrication of organic field effect transistors (OFETs) and organic light emitting diodes (OLED).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Emge, T.J.; Bryden, W.A.; Wiygul, F.M.; Cowan, W.O.; Kistenmacher, T.J.
J. Chem. Phys. , 77, 3188-3188 (1982)
Single-crystal organic field-effect transistors based on dibenzo-tetrathiafulvalene
Mas-Torrent M, et al.
Applied Physics Letters, 86(1), 012110-012110 (2005)
Polymorphism in the 1: 1 Charge-Transfer Complex DBTTF-TCNQ and Its Effects on Optical and Electronic Properties
Goetz KP, et al.
Advanced Electronic Materials, 2(10), 1600203-1600203 (2016)
María Elena Sánchez-Vergara et al.
Molecules (Basel, Switzerland), 20(12), 21037-21049 (2015-11-28)
Sandwich structures were fabricated by a vacuum deposition method using MPc (M = Cu, Zn), with a Tetrathiafulvalene (TTF) derivative, and Indium Tin Oxide (ITO) and aluminum electrodes. The structure and morphology of the deposited films were studied by IR
María Elena Sánchez-Vergara et al.
Polymers, 12(1) (2019-12-22)
Chemical degradation is a major disadvantage in the development of organic semiconductors. This work proposes the manufacture and characterization of organic semiconductor membranes in order to prevent semiconductor properties decreasing. Semiconductor membranes consisting of Nylon-11 and particles of π-conjugated molecular
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![4-Sulfocalix[4]arene ≥97.0% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/850/271/811854c6-ae9d-4f7a-b9e8-c34a7de4e4c6/640/811854c6-ae9d-4f7a-b9e8-c34a7de4e4c6.png)