684538
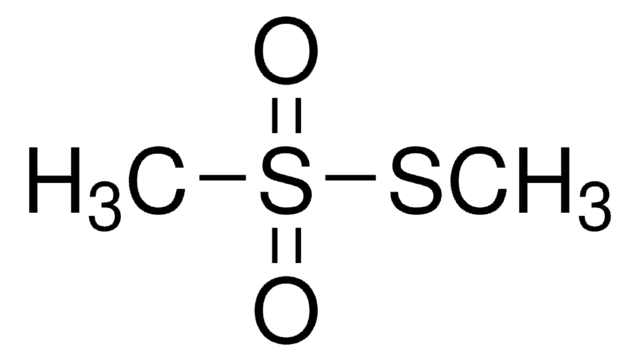
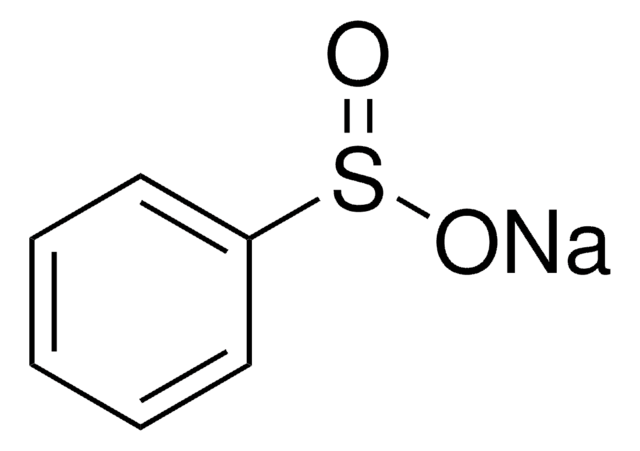
Sodium methanethiosulfonate
95%
Synonym(s):
Methanesulfonothioic acid sodium salt, Sodium methylthiosulfonate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
CH3O2S2Na
CAS Number:
Molecular Weight:
134.15
EC Number:
MDL number:
UNSPSC Code:
12352001
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
crystals
mp
265 °C (dec.)
SMILES string
CS(=O)(=O)S[Na]
InChI
1S/CH4O2S2.Na/c1-5(2,3)4;/h1H3,(H,2,3,4);/q;+1/p-1
InChI key
JFTZUZWJGUCSTE-UHFFFAOYSA-M
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Fabian R Reimold et al.
Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology, 28(3), 435-450 (2011-11-26)
SLC26A4/PDS mutations cause Pendred Syndrome and non-syndromic deafness. but some aspects of function and regulation of the SLC26A4 polypeptide gene product, pendrin, remain controversial or incompletely understood. We have therefore extended the functional analysis of wildtype and mutant pendrin in
Takashi Uehara et al.
Nitric oxide : biology and chemistry, 25(2), 108-111 (2010-11-30)
S-nitrosylation is a well-characterized reaction involving the covalent binding of nitric oxide (NO) to cysteine residues (Cys) in a protein. Similar to protein phosphorylation, S-nitrosylation is a post-translational modification involved in the regulation of a large number of intracellular functions
Jamie S Park et al.
Molecular pharmacology, 80(4), 735-746 (2011-07-28)
Inhibitor and substrate interactions with equilibrative nucleoside transporter 1 (ENT1; SLC29A1) are known to be affected by cysteine-modifying reagents. Given that selective ENT1 inhibitors, such as nitrobenzylmercaptopurine riboside (NBMPR), bind to the N-terminal half of the ENT1 protein, we hypothesized
Rebecca J Howard et al.
Proceedings of the National Academy of Sciences of the United States of America, 108(29), 12149-12154 (2011-07-07)
Despite its long history of use and abuse in human culture, the molecular basis for alcohol action in the brain is poorly understood. The recent determination of the atomic-scale structure of GLIC, a prokaryotic member of the pentameric ligand-gated ion
Vitali Zielke et al.
Acta crystallographica. Section C, Crystal structure communications, 64(Pt 11), o586-o589 (2008-11-08)
The title compound, C(10)H(18)NO(3)S(2), which finds application as a spin label, has triclinic (P\overline{1}) symmetry at 100 (2) K with two independent molecules in the asymmetric unit. Both molecules are very similar with respect to bond lengths and angles, but
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service