All Photos(1)
About This Item
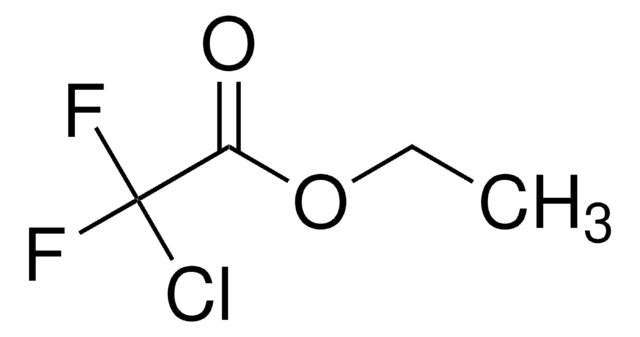
Linear Formula:
Cl(F)CHCO2C2H5
CAS Number:
Molecular Weight:
140.54
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.396 (lit.)
bp
133 °C (lit.)
density
1.212 g/mL at 25 °C (lit.)
SMILES string
CCOC(=O)C(F)Cl
InChI
1S/C4H6ClFO2/c1-2-8-4(7)3(5)6/h3H,2H2,1H3
InChI key
WUHVJSONZHSDFC-UHFFFAOYSA-N
Application
Ethyl chlorofluoroacetate may be used in the synthesis of:
- chlorofluoroacetamide
- ethyl α-fluoro silyl enol ether
- chlorofluoroacetyl chloride
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
125.0 °F - closed cup
Flash Point(C)
51.67 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
One-step method for converting esters to acyl chlorides.
Middleton WJ.
The Journal of Organic Chemistry, 44(13), 2291-2292 (1979)
Xiao-Ting Huang et al.
The Journal of organic chemistry, 67(10), 3231-3234 (2002-05-11)
Ethyl alpha-fluoro silyl enol ether is stereoselectively synthesized in high yield from inexpensive chlorofluoroacetate and Mg (or Zn) in DMF (or HMPA). Lewis acid promoted aldol reaction of this enol ether with aldehydes and ketones gives alpha-fluoro-beta-hydroxy esters in good
The preparation of some derivatives of chlorofluoroacetic acid.
Young JA and Tarrant P.
Journal of the American Chemical Society, 71(1), 3278-3285 (1949)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service