526304
Cyanoborohydride, polymer supported
macroporous, 20-50 mesh, extent of labeling: ~2.0-3.5 mmol/g loading
Synonym(s):
Cyanoborohydride on Amberlite™ IRA-400
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
form
macroporous
Quality Level
reaction suitability
reaction type: solution phase peptide synthesis
reactivity: imine reactive
extent of labeling
~2.0-3.5 mmol/g loading
particle size
20-50 mesh
mp
>300 °C (lit.)
storage temp.
2-8°C
General description
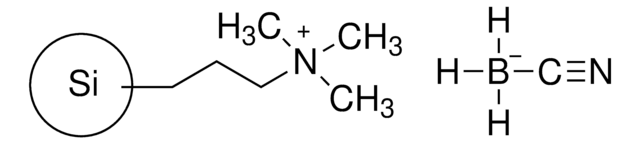
Cyanoborohydride, polymer supported: is a polymer-supported reducing agent used in reductive amination reactions at room temperature.
Application
Polymer supported cyanoborohydride (PSCBH) has been used in the reductive alkylation aminocyclitols for the preparation of corresponding secondary amines.
It can also be used:
It can also be used:
- In combination with manganese dioxide (MnO2) for one-pot conversion of alcohols to secondary or tertiary amines.
- In the synthesis of natural products, (±)-oxomaritidine and (±)-epimaritidine.
Legal Information
Amberlite is a trademark of DuPont de Nemours, Inc.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
In situ oxidation- imine formation- reduction routes from alcohols to amines
Blackburn L and Taylor RJK
Organic Letters, 3(11), 1637-1639 (2001)
Small-Scale One-Pot Reductive Alkylation of Unprotected Aminocyclitols with Supported Reagents
Sisa M, et al.
Synthesis, 2008(19), 3167-3170 (2008)
Synthesis of the alkaloids (?)-oxomaritidine and (?)-epimaritidine using an orchestrated multi-step sequence of polymer supported reagents
Ley S, et al.
Journal of the Chemical Society. Perkin Transactions 1, 3(10), 1251-1252 (1999)
Ley, S.V.
J. Chem. Soc., Perkin Trans., 2239-2239 (1998)
Hutchins, R.O. et al.
Journal of the Chemical Society. Chemical Communications, 1088-1088 (1978)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service