392189
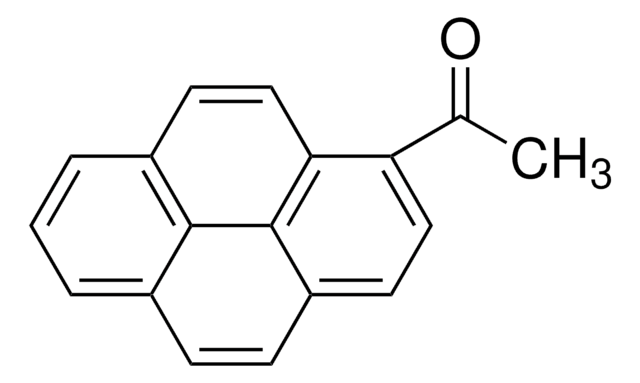
1-Pyreneacetic acid
97%
Synonym(s):
(1-Pyrenyl)acetic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C18H12O2
CAS Number:
Molecular Weight:
260.29
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
210-212 °C (dec.) (lit.)
SMILES string
OC(=O)Cc1ccc2ccc3cccc4ccc1c2c34
InChI
1S/C18H12O2/c19-16(20)10-14-7-6-13-5-4-11-2-1-3-12-8-9-15(14)18(13)17(11)12/h1-9H,10H2,(H,19,20)
InChI key
SDJCLYBBPUHKCD-UHFFFAOYSA-N
General description
1-Pyreneacetic acid is a negatively charged pyrene derivative. It has been proposed as titrating reagent for the standardization titration of Grignard reagents and n-butyl lithium (n-BuLi).
Application
1-Pyreneacetic acid is suitable for use in the following studies:
- Synthesis of N-(2-(methylthio)ethyl)-2-(pyren-1-yl)acetamide, a pyrene amide based Pd2+ probe.
- Synthesis of pyrene-modified β-cyclodextrin.
- To functionalize single walled carbon nanotube field effect transistors (CNT FETs).
- As an agent for characterizing grafting degrees and reactivity of the ester functionalized polypropylenes.
- Synthesis sawhorse-type diruthenium tetracarbonyl complexes.
- Synthesis of (±)-2-(1-pyrenyl)propionic acid, a chiral carboxylic acid.
- Reversible noncovalent functionalization of single walled carbon nanotubes (SWNTs).
- Preparation of peptide nucleic acid (PNA) probes.
- As an internal reference compound in the assessment of solid phase reaction by HPLC-UV.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Fushen Lu et al.
Langmuir : the ACS journal of surfaces and colloids, 26(10), 7561-7564 (2010-01-06)
An effective purification method for single-walled carbon nanotubes (SWNTs) based on a combination of oxidative acid treatment and reversible noncovalent functionalization with 1-pyreneacetic acid is reported. The functionalization was selective toward the nanotubes, allowing a nearly complete removal of residual
Sawhorse-type diruthenium tetracarbonyl complexes derived from pyrenyl-carboxylic acids.
Johnpeter JP and Therrien B.
Inorgorganica Chimica Acta, 405, 437-443 (2013)
Jan Spengler et al.
ACS combinatorial science, 15(5), 229-234 (2013-03-26)
Here we evaluated the use of internal reference compounds for the rapid assessment of reactions performed in solid-phase. An internal reference compound (commercially available) was bound to the resin, together with the substrate, and cleaved with the products after completion
Gabriela Ramos Chagas et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 18(23), 3429-3436 (2017-09-01)
A smart stimuli-responsive surface was fabricated by the electro-copolymerization of pyrene monomers followed by base and acid treatment. Copolymers of pyrenes bearing fluorinated chains (Py-nF
Ali Khalil et al.
Polymers, 12(8) (2020-08-06)
Hydrophobic and amphiphilic derivatives of the biocompatible and biodegradable poly(dimethylmalic acid) (PdiMeMLA), varying by the nature of the lateral chains and the length of each block, respectively, have been synthesized by anionic ring-opening polymerization (aROP) of the corresponding monomers using
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service