389137
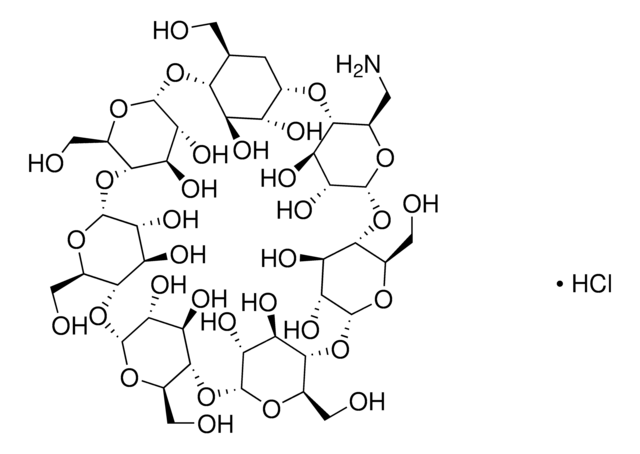
(2-Hydroxyethyl)-β-cyclodextrin
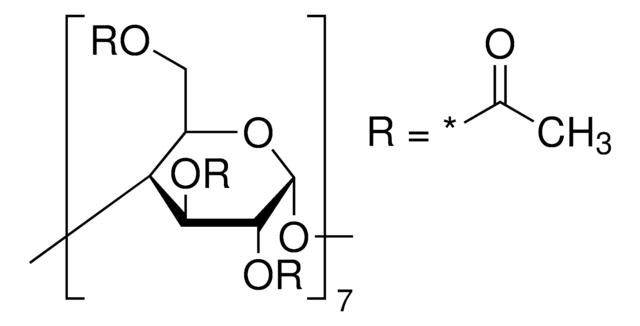
extent of labeling: ~0.7 mol per mol cellulose
Synonym(s):
2-HE-β-CD, 2-hydroxyethyl-β-CD, HP-β-CD
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
form
powder
optical activity
[α]20/D 120 to 140°, c = 1 in H2O
extent of labeling
~0.7 mol per mol cellulose
mp
260 °C (dec.) (lit.)
Looking for similar products? Visit Product Comparison Guide
General description
(2-Hydroxyethyl)-β-cyclodextrin is a chemically modified, hydroxyalkylated derivative of cyclodextrin that finds potential use mainly in cosmetics application.
Application
(2-Hydroxyethyl)-β-cyclodextrin may be used as a chiral selector to resolve cathinone derivatives, lactic acid and oxybutynin enantiomers by capillary electrophoresis (CE), and high performance liquid chromatography (HPLC) techniques.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Cyclodextrin Fundamentals, Reactivity and Analysis
Environmental Chemistry for a Sustainable World (2018)
Design of Nanostructures for Versatile Therapeutic Applications
Pharmaceutical Nanotechnology (2018)
Paweł Mateusz Nowak et al.
Electrophoresis, 39(19), 2406-2409 (2018-07-13)
Methcathinone (ephedrone), 4-methylmethcathinone (mephedrone), and 3-methylmethcathinone (metaphedrone) are toxicologically-important cathinone derivatives used commonly as designer drugs. In this work we show the first method allowing to separate simultaneously all these molecules in a chiral medium, ensuring good resolution between all
L Saavedra et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 766(2), 235-242 (2002-02-05)
The optimization of the separation conditions of the two optical isomers of lactic acid by a factorial design is reported. Initially, different chiral selectors were systematically investigated and then a experimental design with three quantitative factors (cyclodextrin concentration and background
Equilibrium studies on enantioselective extraction of oxybutynin enantiomers by hydrophilic beta-cyclodextrin derivatives
Tang K, et al.
AIChE Journal, 57(11), 3027-3036 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service