368954
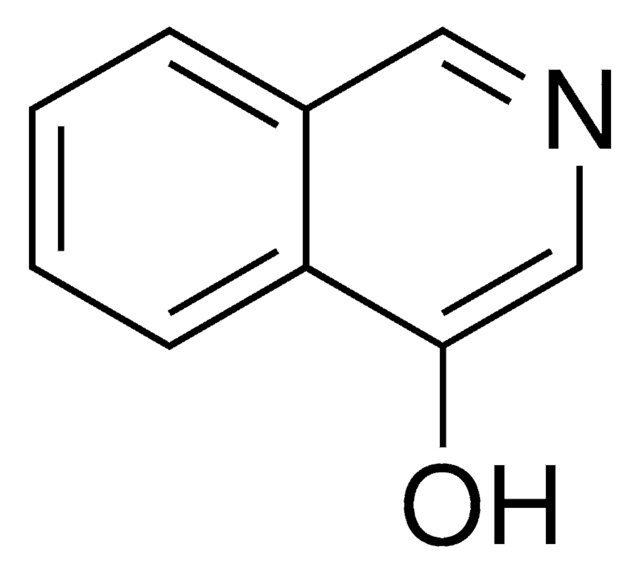
3-Hydroxyisoquinoline
99%
Synonym(s):
3-Isoquinolinol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H7NO
CAS Number:
Molecular Weight:
145.16
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
mp
192-194 °C (lit.)
SMILES string
Oc1cc2ccccc2cn1
InChI
1S/C9H7NO/c11-9-5-7-3-1-2-4-8(7)6-10-9/h1-6H,(H,10,11)
InChI key
GYPOFOQUZZUVQL-UHFFFAOYSA-N
General description
Two excited state proton transfer mechanisms of 3-hydroxyisoquinoline (3HIQ) in cyclohexane and acetic acid has been investigated by time-dependent density functional theory (TDDFT). Spectral and photo physical properties of 3-HIQ in various protic/aprotic solvents were studied. Phototautomerization of 3-HIQ has been reported. Oxo-hydroxy tautomerism and phototautomerism of 3-HIQ has been studied using the matrix-isolation technique.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Anna Gerega et al.
The journal of physical chemistry. A, 111(23), 4934-4943 (2007-05-22)
Oxo-hydroxy tautomerism and phototautomerism of 2-quinolinone, 1-isoquinolinone, 3-hydroxyisoquinoline, 2-quinoxalinone, and 4-quinazolinone were studied using the matrix-isolation technique. These compounds contain a benzene ring fused with a heterocyclic ring of 2-pyridinone, 2-pyrazinone, or 4-pyrimidinone. It turned out that direct attachment of
Neeraj Kumar Joshi et al.
Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 13(6), 929-938 (2014-04-15)
In the present work we report the spectral and photophysical properties of 3-hydroxyisoquinoline in various protic/aprotic solvents. Our steady state and time resolved fluorescence data indicates that in the monomer form of 3HIQ phototautomerization can take place in the excited
Jiajia Guo et al.
Organic & biomolecular chemistry, 13(4), 1179-1186 (2014-11-28)
An efficient and enantioselective strategy to synthesize benzoindolizidines from α,β-unsaturated amino ketones via domino intramolecular aza-Michael addition/alkylation was developed. These reactions were enabled by cinchona alkaloid-derived quaternary ammonium salts as the phase-transfer catalyst. A variety of benzoindolizidines were prepared in
Jinfeng Zhao et al.
Physical chemistry chemical physics : PCCP, 17(2), 1142-1150 (2014-11-25)
Two excited state proton transfer mechanisms of 3-hydroxyisoquinoline (3HIQ) in cyclohexane and acetic acid (ACID) were investigated based on the time-dependent density functional theory (TDDFT), suggesting a different double-proton transfer mechanism from the one proposed previously (J. Phys. Chem. B
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service