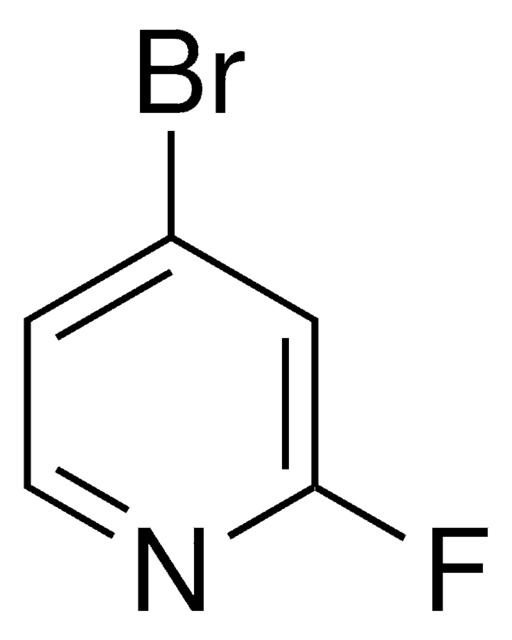
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H6N2
CAS Number:
Molecular Weight:
118.14
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.626 (lit.)
bp
103 °C/1 mmHg (lit.)
density
1.165 g/mL at 25 °C (lit.)
SMILES string
c1ccn2ccnc2c1
InChI
1S/C7H6N2/c1-2-5-9-6-4-8-7(9)3-1/h1-6H
InChI key
UTCSSFWDNNEEBH-UHFFFAOYSA-N
Related Categories
General description
In vivo anti-trypanosomal activity of imidazo[1,2-a]pyridiness in the STIB900 mouse model has been investigated.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mohamed A Ismail et al.
Bioorganic & medicinal chemistry, 16(2), 683-691 (2007-11-03)
The key dinitrile intermediates 4a-d were synthesized by reaction of phenacyl bromide 1 and the appropriate 2-amino-5-bromopyridines to yield 3a-d. Suzuki coupling of 3a-d with 4-cyanophenylboronic acid yielded the 2,6-bis(4-cyanophenyl)-imidazo[1,2-a]pyridine derivatives 4a-d. The bis-amidoximes 5a-d, obtained from 4a-d by the
Richard Ducray et al.
Bioorganic & medicinal chemistry letters, 21(16), 4698-4701 (2011-07-22)
We disclose a novel series of insulin-like growth factor-1 receptor kinase inhibitors based on the 3-(pyrimidin-4-yl)-imidazo[1,2-a]pyridine scaffold. The influence on the inhibitory activity of substitution on the imidazopyridine and at the C5 position of the pyrimidine is discussed. In the
Hongpeng Sun et al.
The Journal of organic chemistry, 77(23), 10745-10751 (2012-11-22)
An efficient tandem route to the synthesis of 3H-1,2a(1),3-triazaacenaphthylene derivatives of the cyclazine family has been developed. Target compounds were obtained in moderate to good yields by a Yb(OTf)(3)/Ag(2)CO(3)-catalyzed, three-component domino reaction. This in turn will set the stage for
Richard Ducray et al.
Bioorganic & medicinal chemistry letters, 21(16), 4702-4704 (2011-07-19)
Following the discovery of imidazopyridine 1 as a potent IGF-1R tyrosine kinase inhibitor, the aniline part has been modified with the aim to optimize the properties of this series. The structure-activity relationships against IGF-1R kinase activity as well as inhibition
Garrett C Moraski et al.
Bioorganic & medicinal chemistry, 20(7), 2214-2220 (2012-03-07)
Tuberculosis (TB) is a devastating disease resulting in a death every 20s. Thus, new drugs are urgently needed. Herein we report ten classes of compounds-oxazoline, oxazole, thiazoline, thiazole, pyrazole, pyridine, isoxazole, imidazo[1,2-a]pyridine, imidazo[1,2-a]pyrimidine and imidazo[1,2-c]pyrimidine-which have good (micromolar) to excellent
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![2-phenylimidazo[1,2-a]pyridine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/281/247/6c2550a0-2f0c-4866-83d8-3c1fb039e165/640/6c2550a0-2f0c-4866-83d8-3c1fb039e165.png)
![Imidazo[1,2-a]pyrazine 97%](/deepweb/assets/sigmaaldrich/product/structures/370/804/1712d71f-52fb-4758-9a22-85b6c96cd4e8/640/1712d71f-52fb-4758-9a22-85b6c96cd4e8.png)
![Imidazo[1,2-a]pyrimidine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/187/001/4862c14e-bec7-4475-85a5-f178e48ff60f/640/4862c14e-bec7-4475-85a5-f178e48ff60f.png)