All Photos(1)
About This Item
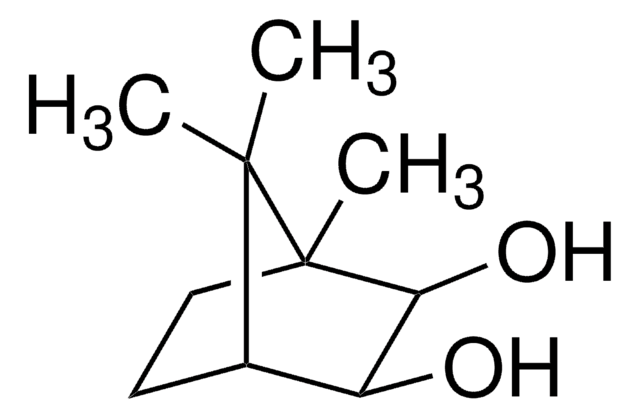
Empirical Formula (Hill Notation):
C10H16O
CAS Number:
Molecular Weight:
152.23
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.469 (lit.)
bp
102-103 °C/50 mmHg (lit.)
density
0.964 g/mL at 25 °C (lit.)
SMILES string
CC1(C)[C@H]2CC3OC3(C)[C@@H]1C2
InChI
1S/C10H16O/c1-9(2)6-4-7(9)10(3)8(5-6)11-10/h6-8H,4-5H2,1-3H3/t6-,7-,8?,10?/m1/s1
InChI key
NQFUSWIGRKFAHK-BGPATTHWSA-N
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
150.8 °F - closed cup
Flash Point(C)
66 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Denis Linares et al.
Bioresource technology, 99(11), 4590-4596 (2007-09-15)
The feasibility of trans-2-methyl-5-isopropylhexa-2,5-dienoic acid (novalic acid) accumulation using the alpha-pinene degradation pathway of Pseudomonas rhodesiae CIP 107491 was studied. This appeared possible by using concentrated living bacterial cells produced under oxygen limitation with alpha-pinene as sole carbon source. The
H Zorn et al.
Journal of biotechnology, 107(3), 255-263 (2004-01-23)
When submerged cultured Pseudomonas fluorescens NCIMB 11761 was fed-batch supplemented with alpha-pinene oxide, a rapid formation of 2,6-dimethyl-5-methylene-hept-(2Z)-enal (I) (isonovalal) was observed. Biotransformation and isomerisation of (I) to the (2E)-isomer (II) (novalal) were enhanced by Lewatit OC 1064, a macroporous
Series: 'Current issues in mutagenesis and carcinogenesis', No. 25.
P A Lefevre
Mutation research, 260(1), 5-7 (1991-05-01)
Hendrik Schewe et al.
Applied microbiology and biotechnology, 78(1), 55-65 (2007-12-07)
Escherichia coli BL21, expressing a quintuple mutant of P450(BM-3), oxyfunctionalizes alpha-pinene in an NADPH-dependent reaction to alpha-pinene oxide, verbenol, and myrtenol. We optimized the whole-cell biocatalyst by integrating a recombinant intracellular NADPH regeneration system through co-expression of a glucose facilitator
Mass spectrometric studies of DNA adducts from a reaction with terpenoids.
Wolfgang Schrader et al.
Angewandte Chemie (International ed. in English), 43(48), 6657-6660 (2004-12-14)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service