215376
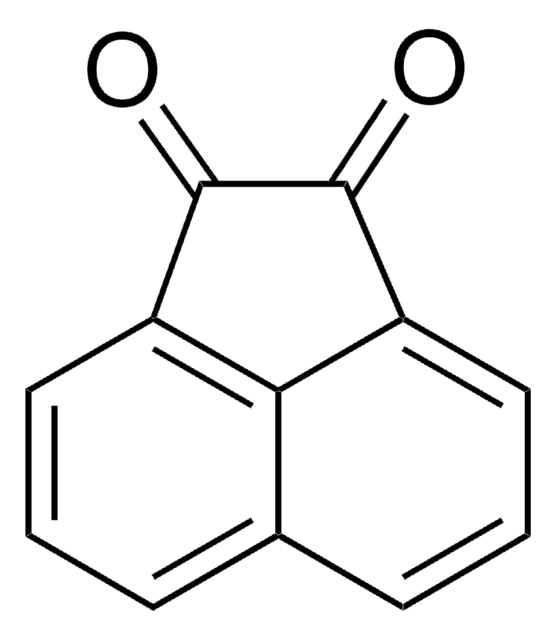
Acenaphthene
99%
Synonym(s):
1,8-Ethylenenaphthalene
About This Item
Recommended Products
vapor density
5.32 (vs air)
Quality Level
vapor pressure
10 mmHg ( 131 °C)
Assay
99%
form
solid
bp
279 °C (lit.)
mp
90-94 °C (lit.)
solubility
chloroform: soluble 5%, clear, colorless to faintly yellow
SMILES string
C1Cc2cccc3cccc1c23
InChI
1S/C12H10/c1-3-9-4-2-6-11-8-7-10(5-1)12(9)11/h1-6H,7-8H2
InChI key
CWRYPZZKDGJXCA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Bacterial oxidation of acenaphthene by Beijerinckia sp. and Beijerinckia sp. strain B8/36 has been reported. Acenapthene forms 1:1:1 inclusion compound by complexing with inclusion compound of β-cyclodextrin and alcohol. Kinetics of the atmospherically important gas-phase reactions of acenaphthene with OH and NO3 radicals, O3 and N2O5 has been investigated at 296 ± 2K.
Application
- Optimization of Acenaphthene Production: A research article demonstrated the use of high-efficiency HILIC capillary columns for slurry packing at 2100 bar, showcasing an advanced technique that could optimize the synthesis and purification of complex polycyclic aromatic hydrocarbons like acenaphthene, essential for enhancing productivity and purity in chemical processes (Anderson et al., 2024).
- Environmental Impact Assessment: A study investigated the relationship between polycyclic aromatic hydrocarbons (PAHs) and reactive oxygen species in PM2.5 emissions, providing a critical evaluation of the environmental impacts of compounds like acenaphthene in industrial regions, thus informing pollution control and environmental safety strategies (Xu et al., 2024).
- Advances in Organic Semiconductor Materials: Research focused on the synthesis and properties of pyrene-bridged acenaphthenes, contributing to the development of novel organic semiconductor materials, which are crucial for electronic and photonic applications, thus broadening the utility of acenaphthene in high-tech industries (Polkaehn et al., 2023).
- Bioassays for Organic Pollutants: Researchers developed bioassays for organic pollutants, including acenaphthene, using chemical activity-based loading of artificial sediments. This approach offers a new method to assess ecological risks associated with organic contaminants, while also optimizing the derivation of predicted no-effect concentrations (PNECs) for polycyclic aromatic hydrocarbons, critical for regulatory compliance and environmental health. (Abel et al., 2024), (Sun et al., 2023).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
257.0 °F - closed cup
Flash Point(C)
125.0 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service