146242
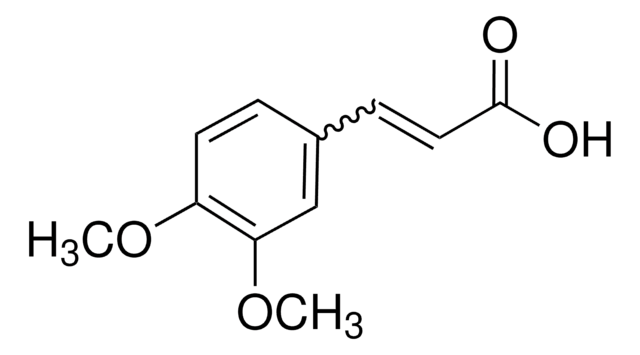
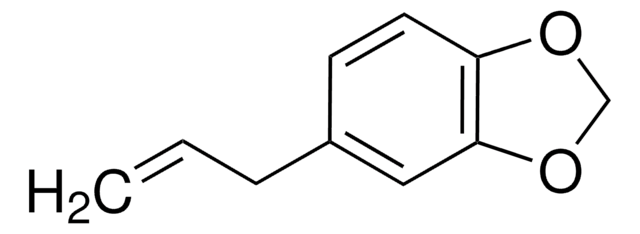
3,4-(Methylenedioxy)cinnamic acid, predominantly trans
99%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H8O4
CAS Number:
Molecular Weight:
192.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
powder
mp
242-244 °C (dec.) (lit.)
SMILES string
OC(=O)\C=C\c1ccc2OCOc2c1
InChI
1S/C10H8O4/c11-10(12)4-2-7-1-3-8-9(5-7)14-6-13-8/h1-5H,6H2,(H,11,12)/b4-2+
InChI key
QFQYZMGOKIROEC-DUXPYHPUSA-N
Related Categories
General description
3,4-(Methylenedioxy)cinnamic acid is an inhibitor of the phenylpropanoid enzyme 4-hydroxycinnamoyl-CoA ligase. It undergoes electron transfer reaction with trichloromethylperoxyl radical and reaction has been studied by pulse radiolysis.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rogério Barbosa Lima et al.
PloS one, 8(12), e80542-e80542 (2013-12-07)
Cinnamic acid and its hydroxylated derivatives (p-coumaric, caffeic, ferulic and sinapic acids) are known allelochemicals that affect the seed germination and root growth of many plant species. Recent studies have indicated that the reduction of root growth by these allelochemicals
J X Pan et al.
Free radical research, 30(3), 241-245 (2000-03-11)
The electron transfer reactions between the trichloromethylperoxyl radical (Cl3COO*) and hydroxycinnamic acid derivatives, including chlorogenic acid, sinapic acid, caffeic acid, ferulic acid and 3,4-(methylenedioxy)cinnamic acid, have been studied by pulse radiolysis. The hydroxycinnamic acid derivatives, especially sinapic acid, are identified
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service