135321
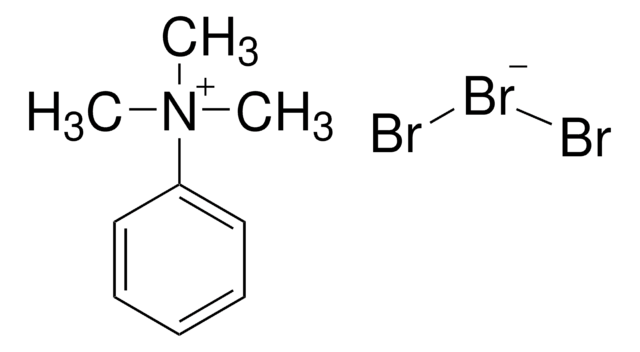
Trimethylphenylammonium bromide
98%
Synonym(s):
Phenyltrimethylammonium bromide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)3N(Br)C6H5
CAS Number:
Molecular Weight:
216.12
Beilstein:
3917006
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
technique(s)
titration: suitable
mp
215 °C (dec.) (lit.)
SMILES string
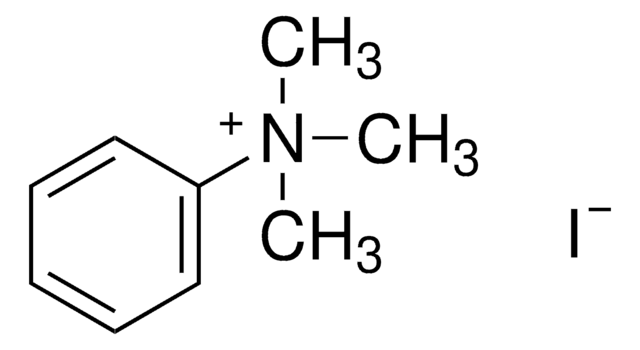
[Br-].C[N+](C)(C)c1ccccc1
InChI
1S/C9H14N.BrH/c1-10(2,3)9-7-5-4-6-8-9;/h4-8H,1-3H3;1H/q+1;/p-1
InChI key
GNMJFQWRASXXMS-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A Ranz et al.
Journal of biochemical and biophysical methods, 69(1-2), 3-14 (2006-03-28)
In the present study, a derivatization method for the determination of acidic herbicides has been investigated. This procedure involves the methylation with the quaternary ammonium salt trimethylanilinium hydroxide (TMAH) directly in the gas chromatographic auto-sampler vial for analysis by gas
Timothy J Jensen et al.
Journal of photochemistry and photobiology. B, Biology, 100(2), 100-111 (2010-06-19)
Five cationic porphyrins bearing one to four -N(CH(3))(3)(+) groups linked to the p-phenyl positions of 5,10,15,20-tetraphenylporphyrin (TPP) were synthesized in order to study the effect of overall charge and its distribution on the cellular uptake, phototoxicity and intracellular localization using
Petr Gebauer et al.
Electrophoresis, 29(5), 1067-1076 (2008-01-26)
In electrophoresis, the introduction of a sample into the BGE creates two new zone boundaries, the front and rear boundary of the sample zone which is sandwiched between the BGE. During an electrophoresis run, these boundaries move and split into
Y El-Nahhal et al.
Journal of agricultural and food chemistry, 48(10), 4791-4801 (2000-10-29)
This study aimed to design formulations of hydrophobic herbicides, alachlor and metolachlor, by adsorbing them on the clay mineral montmorillonite preadsorbed by the small organic cation phenyltrimethylammonium (PTMA). An adsorption model that considers electrostatics and specific binding and the possibility
Neera Singh
Journal of environmental science and health. Part. B, Pesticides, food contaminants, and agricultural wastes, 41(1), 17-29 (2006-01-06)
The study aims to prepare the organoclay complexes of metolachlor and metribuzin so as to reduce their downward mobility in soil profile. The organoclays were preadsorbed with phenyltrimethylammonium (PTMA) (50% of cation exchange capacity [CEC]) and hexadecyltrimethylammonium (HDTMA) (100% of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service